H-89 DIHYDROCHLORIDE HYDRATE
Synonym(s):N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride
- CAS NO.:130964-39-5
- Empirical Formula: C20H20BrN3O2S.2ClH.H2O
- Molecular Weight: 537.304
- MDL number: MFCD00214120
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
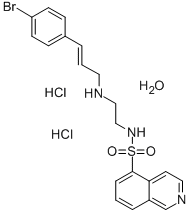
What is H-89 DIHYDROCHLORIDE HYDRATE?
Chemical properties
White Solid
The Uses of H-89 DIHYDROCHLORIDE HYDRATE
H-89 dihydrochloride hydrate has been used in the inhibition of protein kinase A in Leydig cells, primary calvarial osteoblasts (pOBs), rat granulosa cell line (LH-15 cells) and human umbilical vein endothelial cells (HUVECs).
The Uses of H-89 DIHYDROCHLORIDE HYDRATE
Selective inhibitor of Protein Kinase A (cyclic AMP-dependeant Protein Kinase) with an inhibitory constant of 0.0048 uM.
What are the applications of Application
H-89 dihydrochloride is a potent, cell-permeable and reversible inhibitor of PKA
Definition
ChEBI: N-[2-(4-bromocinnamylamino)ethyl]isoquinoline-5-sulfonamide dihydrochloride is a hydrochloride salt prepared from N-[2-(4-bromocinnamylamino)ethyl]isoquinoline-5-sulfonamide and two equivalents of hydrogen chloride. It has a role as an EC 2.7.11.11 (cAMP-dependent protein kinase) inhibitor. It contains a N-[2-(4-bromocinnamylamino)ethyl]isoquinoline-5-sulfonamide(2+).
Biological Activity
h 89 2hcl is a potent pka inhibitor. in a cell-free assay, the ki of h 89 is 48 nm, 10-fold selective for pka than pkg and 500-fold greater selectivity than pkc, mlck, calmodulin kinase ii and casein kinase i/ii [1].[1]. chijiwa t, mishima a, hagiwara m, et al. inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic amp-dependent protein kinase, n-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide (h-89), of pc12d pheochromocytoma cells[j]. journal of biological chemistry, 1990, 265(9): 5267-5272.[2]. lochner a, moolman j a. the many faces of h89: a review[j]. cardiovascular drug reviews, 2006, 24(3‐4): 261-274.[3]. lee t h, linstedt a d. potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-golgi transport revealed by protein kinase inhibitor h89[j]. molecular biology of the cell, 2000, 11(8): 2577-2590.
Biochem/physiol Actions
H-89 dihydrochloride hydrate is a selective inhibitor of protein kinase A (PKA). It also inhibits potassium (K+) current in rat myocytes. It mediates Na+ transport by interacting with α subunits of epithelial Na+ channel (ENaC).
in vitro
in pc12d cells, pretreatment with h-89 dose-dependently inhibited the forskolin-induced protein phosphorylation, with no influence in intracellular cyclic amp levels. in pc12d cells, h-89 significantly inhibited the forskolin-induced neurite outgrowth. in pc12d cells, pretreatment with h-89 (30 μm) strikingly inhibited camp-dependent histone iib phosphorylation activity in cell lysates while showed no effects on other protein phosphorylation activity such as cgmp-dependent histone iib phosphorylation activity [1]. h 89 was a potent and selective pka inhibitor with ki of 48 nm in a cell-free assay [2]. h89 also inhibited s6k1, msk1, pka, rockii, pkbα and mapkap-k1b kinases with ic50 of 80, 120, 135, 270, 2600 and 2800 nm, respectively [2]. in the hypotonic medium, 50 μm h89, a concentration commonly used to inhibit pka, prevented the redistribution response. in normal medium, h89 (50 μm) induced the redistribution of ergic 53 to the er by 20 min [3].
storage
-20°C
Properties of H-89 DIHYDROCHLORIDE HYDRATE
| Melting point: | 195-200°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: ≥10mg/mL |
| form | powder |
| color | off-white |
Safety information for H-89 DIHYDROCHLORIDE HYDRATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for H-89 DIHYDROCHLORIDE HYDRATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![N-(2-[P-BROMOCINNAMYLAMINO]ETHYL)-5-ISOQUINOLINESULFONAMIDE HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/127243-85-0.gif)
You may like
-
 H-89 dihydrochloride hydrate CAS 130964-39-5View Details
H-89 dihydrochloride hydrate CAS 130964-39-5View Details
130964-39-5 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1