Sodium carbonate decahydrate
Synonym(s):Sal soda;Soda;Soda, Sodium Carbonate decahydrate;Sodium carbonate decahydrate
- CAS NO.:6132-02-1
- Empirical Formula: CH20Na2O13
- Molecular Weight: 286.14
- MDL number: MFCD00149178
- EINECS: 612-116-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:24:44
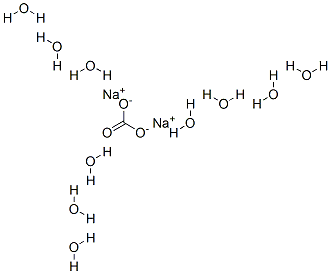
What is Sodium carbonate decahydrate?
Chemical properties
Sodium carbonate decahydrate is known as sal soda or washing soda. Its chemical formula is Na2CO3.10H2O. Anhydrous sodium carbonate, Na2CO3, is commonly called soda ash. Sodium carbonate is a white, almost white, or colorless inorganic salt, produced as crystalline powder or granules. It is hygroscopic and odorless with an alkaline taste. The decahydrate, NaCO3.10H2O (washing soda) is a translucent efflorescence crystalline solid. It can be used in the preparation of eutectic hydrate salts, which can be used to remove phase change materials for thermal energy storage. It can also be dehydrated to generate cold heat using a chemical heat pump.
The Uses of Sodium carbonate decahydrate
Sodium carbonate decahydrate is used as a raw material in various industries like glass, paper making, soap, detergent, textile and leather. It is also used as a food additive. It is also involved as a precipitation agent of carbonate as well as an analytical reagent in laboratories. It plays an important role as strong base and powerful electrolyte. It finds application in metal refining and cement production.
What are the applications of Application
Sodium carbonate decahydrate is a sodium salt of carbonic acid used as a detergent, strong base or a powerful electrolyte
Production Methods
Sodium carbonate is produced by the ammonia-soda process, also known as the Solvay process.
Definition
ChEBI: Sodium carbonate decahydrate is an organooxygen compound.
Pharmaceutical Applications
Sodium carbonate is used as an alkalizing agent in injectable,
ophthalmic, oral, and rectal formulations.
In effervescent tablets or granules, sodium carbonate is used in
combination with an acid, typically citric acid or tartaric acid.
When the tablets or granules come into contact with water, an acid–
base reaction occurs in which carbon dioxide gas is produced and
the product disintegrates. Raw materials with low moisture
contents are required to prevent the early triggering of the
effervescent reaction.
As an alkalizing agent, concentrations of sodium carbonate
between 2% and 5% w/w are used in compressed tablet
formulations. As an effervescent agent, concentrations of
sodium carbonate up to 10% w/w can be used.
Therapeutically, sodium carbonate is also used as an oral
antacid.
Safety
Sodium carbonate is used in injectable, oral, and rectal pharmaceutical
formulations. The pure form of sodium carbonate is mildly
toxic by ingestion, moderately toxic by inhalation and SC routes,
and very toxic by the IP route. It is irritating to the skin and eyes.
Dust and vapors of sodium carbonate may irritate mucous
membranes, causing coughing and shortness of breath. It also has
experimental reproductive effects.
Sodium carbonate can migrate to food from packaging
materials. When used as an excipient or antacid, sodium carbonate
is generally regarded as a nontoxic and nonirritating material.
LD50 (mouse, IP): 0.12 g/kg
LD50 (mouse, SC): 2.21 g/kg
LD50 (rat, oral): 4.09 g/kg
Storage
Sodium carbonate converts to the monohydrate form when in contact with water and produces heat. It begins to lose carbon dioxide at temperatures above 400℃ and decomposes before boiling. Store in airtight containers.
Incompatibilities
Sodium carbonate decomposes when in contact with acids in the presence of water to produce carbon dioxide and effervescence. It may react violently with aluminum, phosphorous pentoxide, sulfuric acid, fluorine, and lithium.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (injections;
ophthalmic solution; oral capsules and tablets; rectal suspensions).
Included in the Canadian List of Acceptable Non-medicinal
Ingredients. Included in parenteral (powder for solution for
injection) and nonparenteral medicines (oral effervescent tablets,
soluble tablets, granules, lozenges, chewing gums) licensed in the
UK.
USP32–NF27 allows either the anhydrous or the monohydrate
form.
Properties of Sodium carbonate decahydrate
| Melting point: | 34 °C |
| Density | 1.46 |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Small Crystals |
| color | White |
| Specific Gravity | 1.46 |
| PH | 11-12 (50g/l, H2O, 25℃) |
| Water Solubility | 210 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,8596 |
| CAS DataBase Reference | 6132-02-1(CAS DataBase Reference) |
Safety information for Sodium carbonate decahydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Sodium carbonate decahydrate
Sodium carbonate decahydrate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 6132-02-1 Sodium carbonate decahydrate 98%View Details
6132-02-1 Sodium carbonate decahydrate 98%View Details
6132-02-1 -
 Sodium Carbonate Decahydrate CAS 6132-02-1View Details
Sodium Carbonate Decahydrate CAS 6132-02-1View Details
6132-02-1 -
 Sodium carbonate decahydrate CAS 6132-02-1View Details
Sodium carbonate decahydrate CAS 6132-02-1View Details
6132-02-1 -
 50 kg Sodium Carbonate Decahydrate Ar/AcsView Details
50 kg Sodium Carbonate Decahydrate Ar/AcsView Details
6132-02-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
