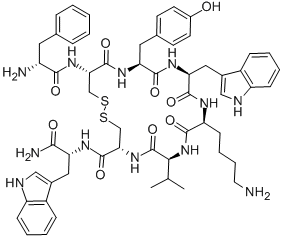
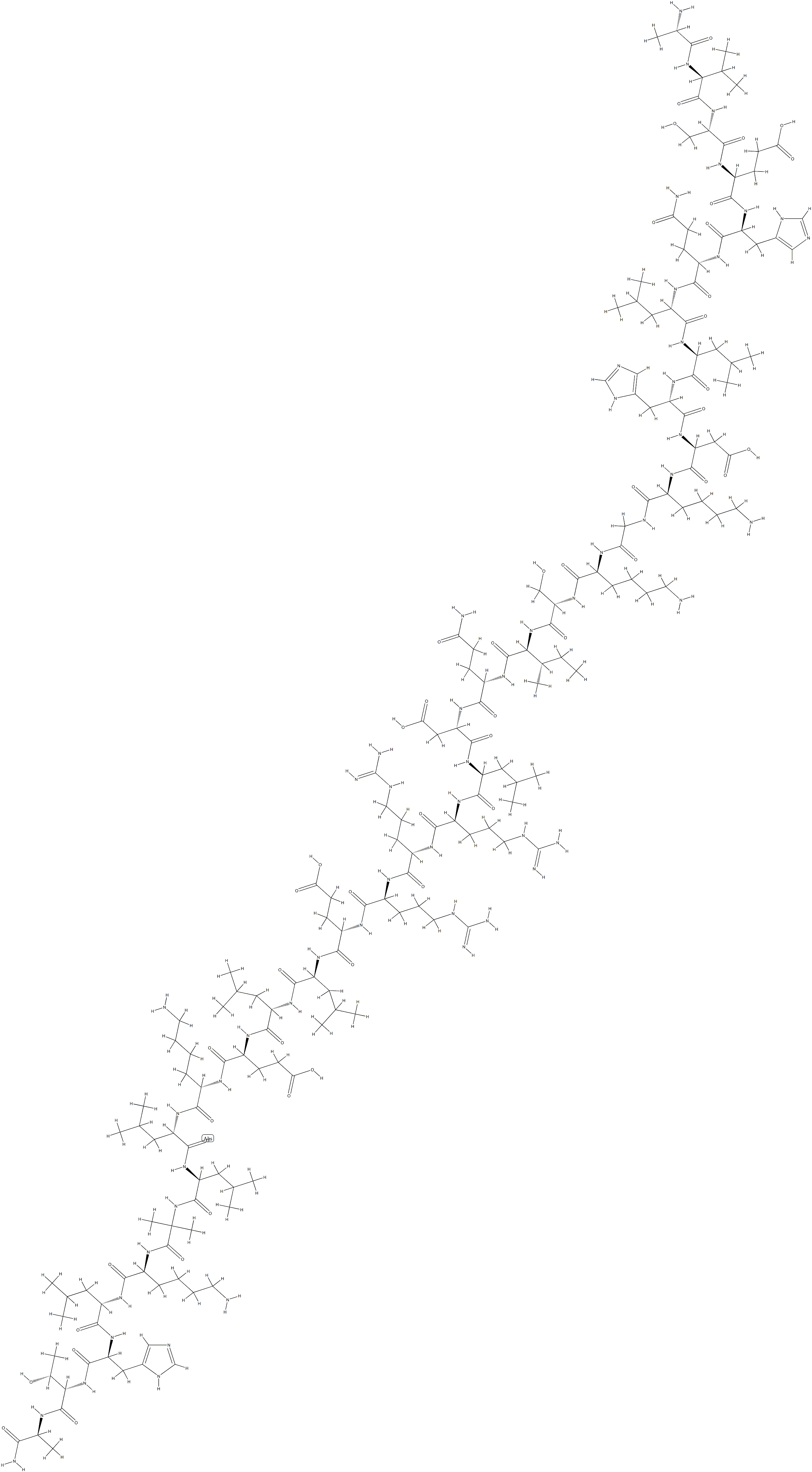
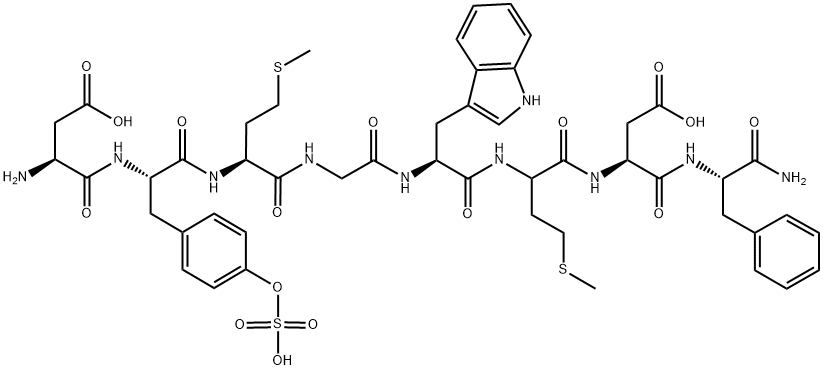
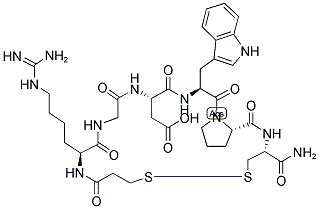
Etelcalcetide
- CAS NO.:1262780-97-1
- Empirical Formula: C38H73N21O10S2
- Molecular Weight: 1048.25
- MDL number: MFCD31735128
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-05 11:59:05
What is Etelcalcetide?
Absorption
The pharmacokinetics of etelcalcetide is linear and does not change over time following single (5 to 60 mg) and multiple intravenous doses (2.5 to 20 mg) in chronic kidney disease patients with secondary hyperparathyroidism requiring hemodialysis. Etelcalcetide exhibited tri-exponential decay following intravenous administration. Based on population pharmacokinetic analysis, following three times a week intravenous dosing at the end of each 3- to 6-hour hemodialysis session in chronic kidney disease patients, etelcalcetide plasma levels reached steady state in 7-8 weeks after dosing with a predicted accumulation ratio of 3- to 4-fold
The Uses of Etelcalcetide
N-Acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl- D-Argininamide-L-cysteine Disulfide is a useful research chemical used in the treatment of chronic kidney disease-??mineral and bone disorder.
Indications
Etelcalcetide is a calcium-sensing receptor agonist indicated for: Secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on hemodialysis.
Background
Etelcalcetide is a calcimimetic drug for the treatment of secondary hyperparathyroidism in patients undergoing hemodialysis. Etelcalcetide was approved (trade name Parsabiv) for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on hemodialysis in February, 2017.
Definition
ChEBI: Etelcalcetide is an oligopeptide.
Pharmacokinetics
Following a single intravenous bolus administration of etelcalcetide, PTH levels decreased within 30 minutes post dose. In the single-dose study, the extent and duration of the reduction in PTH increased with increasing dose. Reduction in PTH levels correlated with plasma etelcalcetide concentrations in hemodialysis patients. The reduction in PTH resulted in reductions in calcium and attenuation of post-dialytic phosphate elevation. The effect of reducing PTH levels was maintained throughout the 6-month dosing period when etelcalcetide was administered by intravenous bolus three times a week.
Clinical Use
Synthetic peptide calcimimetic agent:Treatment of secondary hyperparathyroidism in patients on haemodialysis
Drug interactions
Potentially hazardous interactions with other drugs Avoid with cinacalcet.
Metabolism
Etelcalcetide is not metabolized by CYP450 enzymes. Etelcalcetide is biotransformed in blood by reversible disulfide exchange with endogenous thiols to predominantly form conjugates with serum albumin. Following a single radiolabeled dose of etelcalcetide in chronic kidney disease patients with secondary hyperparathyroidism requiring hemodialysis, the plasma exposure of biotransformation products is approximately 5-fold higher than that of etelcalcetide and their concentration-time course parallels that of etelcalcetide.
Metabolism
Etelcalcetide is biotransformed in blood by reversible disulphide exchange with endogenous thiols to predominantly form conjugate with serum albumin
Properties of Etelcalcetide
| Density | 1.58±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 2.01±0.10(Predicted) |
Safety information for Etelcalcetide
Computed Descriptors for Etelcalcetide
Etelcalcetide manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1