sinapultide
- CAS NO.:138531-07-4
- Empirical Formula: C126H238N26O22
- Molecular Weight: 2469.4
- MDL number: MFCD18251732
- Update Date: 2024-03-28 16:55:20
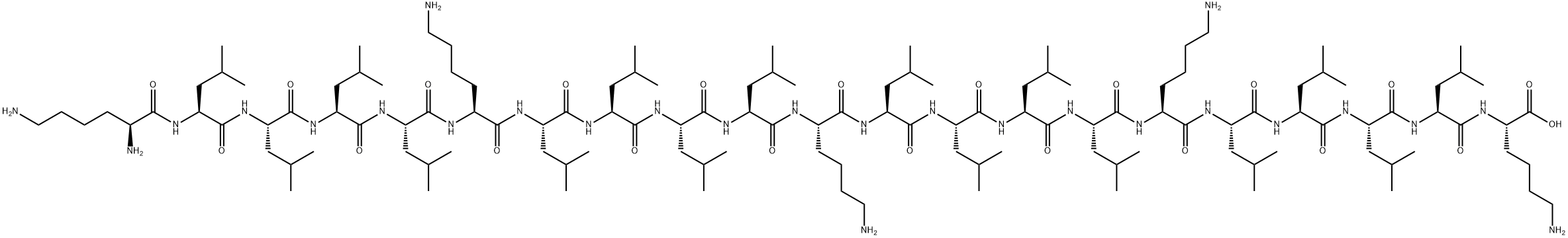
What is sinapultide?
Absorption
Administered directly to the lung, where biophysical effects occur at the terminal airways and alveolar surface. No human pharmacokinetic studies have been done to characterize the absorption, distribution, metabolism, or elimination of this drug .
Toxicity
If respiration, ventilation, or oxygenation is clearly affected after an accidental overdose, aspirate as much of the suspension as possible and provide the infant with supportive treatment .
The Uses of sinapultide
Pulmonary surfactant.
Indications
Infant respiratory distress syndrome , , , .
Background
Sinapultide (also known as KL4 peptide) is a synthetic protein used to mimic human lung surfactant protein B. This protein has a weight of 2469.40.
Sinapultide is a 21-residue peptide made up of lysine (K) and leucine (L) residues with the sequence KLLLLKLLLLKLLLLKLLLLK (KL4), in aqueous dispersion with the phospholipids DPPC (dipalmitoylphosphatidylcholine), POPG (palmitoyloleoyl-phosphatidylglycerol), and palmitic acid, to create the drug lucinactant.
The product was originally developed by the Scripps Research Institute, then licensed to Windtree Therapeutics. Windtree Therapeutics plans a phase III trial for Respiratory distress syndrome in 2018.
Respiratory distress syndrome (RDS) is a major cause of mortality and morbidity in preterm infants. Surfactant replacement therapy has been commonly used to prevent and treat RDS in these newborns and is now a standard of care. First-generation synthetic surfactants that were previously used, such as Exosurf did not contain any surfactant protein. This large disadvantage was overcome with animal-derived surfactant products which contain specific proteins but are limited, but must be derived from animal sources. This has led to the development of newer synthetic surfactants such as lucinactant (Surfaxin), which contains sinapultide. Phase 3 clinical trials with Surfaxin show promising results with similar efficacy as animal-derived surfactants while avoiding the use of animal-origin products.
Windtree is currently developing aerosolized KL4 surfactant to treat RDS in premature infants, and thereafter, to potentially address a range of indications in neonatal, pediatric and adult critical care patient populations.
Pharmacokinetics
Windtree’s KL4 surfactant technology produces a synthetic surfactant that is structurally similar to human pulmonary surfactant and contains a proprietary synthetic peptide KL4 (sinapultide), cost a 21-amino acid peptide that is formulated to mimic the essential attributes of the human surfactant protein B (SP-B). This protein is one of four surfactant proteins and is the most important for the adequate function of the respiratory system. Windtree has demonstrated in pre-clinical studies that KL4 surfactant may possess certain other beneficial properties, including alteration of the inflammatory process, antimicrobial properties as well as non-immunogenicity .
Metabolism
Not Available
Properties of sinapultide
| Boiling point: | 2047.1±65.0 °C(Predicted) |
| Density | 1.093 |
| pka | 3.31±0.10(Predicted) |
Safety information for sinapultide
Computed Descriptors for sinapultide
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8