Thyrotropin-releasing hormone
Synonym(s):TRH;PIN2;TRF;TRF1;TERF1
- CAS NO.:24305-27-9
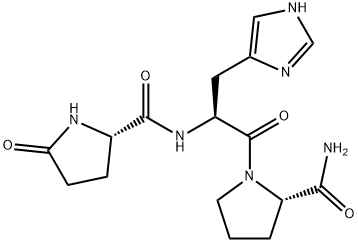
- Empirical Formula: C16H22N6O4
- Molecular Weight: 362.38
- MDL number: MFCD00038640
- EINECS: 246-143-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Thyrotropin-releasing hormone?
Description
The first hypothalamic hypophysiotropic neurohormone identified, TRH consists of the tripeptide pGlu-His-ProNH2. It stimulates the secretion ofthyroid-stimulating hormone (TSH), prolactin (PRL), and growth hormone (GH), and also functions as a neurotransmitter and neuromodulator.
Chemical properties
White or yellowish-white powder, hygroscopic.
The Uses of Thyrotropin-releasing hormone
prothyrotropin
The Uses of Thyrotropin-releasing hormone
Thyrotropin-Releasing Hormone is a hypothalamic hypophysiotropic neuropeptide, which has the ability to stimulate the release of thyroid-stimulating hormone in mammals. It is proven that Thyrotropin-Releasing Hormone can be used to accelerate wound healing.
Background
Protirelin is the pharmaceutically available synthetic analogue of the endogenous peptide thyrotropin-releasing hormone (TRH). It is a tri-peptide tropic hormone, released by the hypothalamus, that stimulates the release of Thyroid Stimulating Hormone (TSH) and prolactin from the anterior pituitary.
Although not currently available in any FDA-approved product, protirelin is a component of the TRH Test where it is used to test the response of the anterior pituitary gland in conditions such as secondary hypothyroidism and acromegaly.
Indications
Thyrotropin-releasing hormone, or protirelin, consists of three amino acids. TRH (Relefact TRH) is used for tests to distinguish primary from secondary hypothyroidism.
Definition
ChEBI: Protirelin is a tripeptide composed of L-pyroglutamyl, L-histidyl and L-prolinamide residues joined in sequence. It has a role as a human metabolite. It is a peptide hormone and a tripeptide.
General Description
Thyrotropin releasing hormone (TRH) is a tripeptide hypothalamic regulatory hormone, encoded by the gene mapped to human chromosome 3q13.3-q21. TRH is expressed in a variety of organs including central nervous system (CNS) and gastrointestinal tract.
Biochem/physiol Actions
Thyrotropin releasing hormone (TRH) stimulates production and secretion of thyrotropin (TSH) and prolactin from the anterior pituitary. It also plays a vital role as a neurotransmitter and neuromodulator.
Clinical Use
TRH (200–500μg) administered intravenously to normal subjects causes a rise in TSH levels within 15–30min, resulting in an increase in T3 levels within 90–150min. In primary hypothyroidism, TSH hyperresponse to TRH occurs, with a typical elevation in the basal TSH levels. In secondary (pituitary) hypothyroidism, an impaired TSH response to TRH occurs, whereas in tertiary (hypothalamic) hypothyroidism normal or increased TSH response to TRH occurs. Protirelin is used to test the response of the anterior pituitary to TRH in people who may have medical conditions of thyroid function, including hyperthyroidism, Graves’ disease, and hypothyroidism. In addition, TRH has been used to assess the ability of the prolactin secretion in the pituitary.
Side Effects
The peak TSH response to intravenous TRH occurs at 20 minutes. The mild and transient side effects, which occur only after intravenous TRH, include nausea, a flushing sensation, a desire to micturate, a peculiar taste, and tightness in the chest[1].
Metabolism
Not Available
Properties of Thyrotropin-releasing hormone
| Melting point: | >143°C (dec.) |
| Boiling point: | 494°C (rough estimate) |
| alpha | -50 (D/25℃) (c=1.5, H2O)-65.5 (D) (c=1.0, H2O) |
| Density | 1.1675 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | −20°C |
| solubility | H2O: 10 mg/mL, clear, colorless |
| form | powder |
| pka | 13.05±0.20(Predicted) |
| color | White to Off-White |
| PH | pH (10g/l, 25℃) : 3.0~4.0 |
| Merck | 13,9663 |
| BRN | 770238 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 24305-27-9 |
Safety information for Thyrotropin-releasing hormone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Thyrotropin-releasing hormone
| InChIKey | ITYONPBTNRIEBA-SRVKXCTJSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![TRH,(His[T-Me])-TRH, [3-Methyl-His2]-Thyrotropin releasing hormone](https://img.chemicalbook.in/CAS/GIF/34367-54-9.gif)
You may like
-
 Thyrotropin releasing hormone CAS 24305-27-9View Details
Thyrotropin releasing hormone CAS 24305-27-9View Details
24305-27-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1