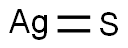Silver
Synonym(s):Silver;Silver nanowire;Silverjet DGH-55HTG;Silverjet DGH-55LT-25C;Silverjet DGP-40LT-15C
- CAS NO.:7440-22-4
- Empirical Formula: Ag
- Molecular Weight: 107.87
- MDL number: MFCD00003397
- EINECS: 231-131-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:07

What is Silver?
Absorption
Although metallic silver is inert in the presence of human tissues, silver and its compounds may dissociate upon contact with skin surface, body fluids, and secretions, allowing the silver ions to be absorbed into the blood circulation . Soluble silver salts are absorbed from the respiratory and gastrointestinal tracts. However, up to 90-99% of orally ingested silver is not absorbed and percutaneous absorption of silver ions through intact or damaged skin is also reported to be low . Absorbed silver ions are deposited into elastic and connective tissues throughout the body . Biologically active silver ions mainly binds to intracellular proteins as inert complexes, and readily binds and precipitates with inorganic cations like chloride and phosphate, which explains low absorption .
Toxicity
Acute oral LD50, acute dermal LD50, and acute inhalation LD50 for 4 hours in rat are >5000 mg/kg, >2000 mg/kg, and >5.16 mg/m^3, respectively .
While individuals experiencing mild to moderate silver toxicity remain asymptomatic, chronic inhalation has been associated with mild chronic bronchitis and rare cases of exposure to large amounts of silver have been associated with symptoms of peripheral neuropathy, decreased mental status, and seizures . Argyria is a detoxification mechanism for excess silver where the body sequesters and deposits excess silver in the blood vessels and connective tissue to render it in the form of silver protein complexes or silver sulphide . The development of argyria through occupational exposure is reported to be a slow process . While argyria does not cause significant pathological damage in any tissue , heavy deposition of insoluble silver precipitates can cause discoloration or blue-grey darkening of the eyes, nasal septum, throat, skin, and other internal organs following repeated exposure . Signs from prolonged intake of low doses of silver compound may include fatty degeneration of the liver and kidneys, and changes in blood cells . Silver may cause metal fume fever, and colloidal silver preparations are known to exert harmful effects in humans .
Description
Silver is one of the earliest known metals. Silver has no known physiologic or biologic function, though colloidal silver is widely sold in health food stores. Silver has high thermal and electrical conductivity and resists oxidation in air that is devoid of hydrogen sulfide.
Chemical properties
Silver is a white lustrous metal that is extremely ductile and malleable. Silver does not oxidize in O2 by heating. It becomes Ag2O3 in O3 and black Ag2S3 in S2 and H2S. It is soluble in HNO3 and concentrated H2SO4 . It is not soluble in alkali.
Chemical properties
Elemental silver has a face-centered cubic crystal structure. Silver is a white metal, softer than copper and harder than gold. When molten, silver is luminescent and occludes oxygen, but the oxygen is released upon solidification. As a conductor of heat and electricity, silver is superior to all other metals. Silver is soluble in HNO3 containing a trace of nitrate; soluble in hot 80% H2SO4; insoluble in HCl or acetic acid; tarnished by H2S, soluble sulfides and many sulfur-containing organic substances (e.g., proteins); not affected by air or H2O at ordinary temperatures, but at 200 C, a slight film of silver oxide is formed; not affected by alkalis, either in solution or fused. There are two stable, naturally occurring isotopes, 107Ag and 109Ag. In addition, there are reported to be 25 less stable isotopes, ranging in half-life from 5 seconds to 253 days.
Physical properties
Silver is located in group 11 (IB) of period 5, between copper (Cu) above it in period 4 andgold (Au) below it in period 6. Thus, silver’s chemical and physical properties are somewhatsimilar to these two group 11 partners.Silver is a soft, while, lustrous metal that can be worked by pounding, drawing througha die, rolling, and so forth. It is only slightly harder than gold. It is insoluble in water, but it will dissolve in hot concentrated acids. Freshly exposed silver has a mirror-like shine thatslowly darkens as a thin coat of tarnish forms on its surface (from the small amount ofnatural hydrogen sulfide in the air to form silver sulfide, AgS). Of all the metals, silver isthe best conductor of heat and electricity. This property determines much of its commercialusefulness. Its melting point is 961.93°C, its boiling point is 2,212°C, and its density is10.50 g/cm3.
Isotopes
There are 59 isotopes of silver, ranging from Ag-93 to Ag-130 with half-livesfrom a few milliseconds to a few days to 418 years. All but two of these 59 isotopes areradioactive and are produced synthetically. The two stable isotopes found in nature areAg-107 and Ag-109. These two make up 100% of the element’s existence in the Earth’scrust.
Origin of Name
Silver’s modern chemical symbol (Ag) is derived from its Latin word argentum, which means silver. The word “silver” is from the Anglo-Saxon world “siolfor.” Ancients who first refined and worked with silver used the symbol of a crescent moon to represent the metal.
Occurrence
Silver is the 66th most abundant element on the Earth, which means it is found at about0.05 ppm in the Earth’s crust. Mining silver requires the movement of many tons of ore torecover small amounts of the metal. Nevertheless, silver is 10 times more abundant than gold.And though silver is sometimes found as a free metal in nature, mostly it is mixed with theores of other metals. When found pure, it is referred to as “native silver.” Silver’s major ores areargentite (silver sulfide, Ag2S) and horn silver (silver chloride, AgCl). However, most silver isrecovered as a by-product of the refining of copper, lead, gold, and zinc ores. Although silveris mined in many countries, including the United States, Mexico, and Canada, most silver isrecovered from the electrolytic processing of copper ores. Silver can also be recovered throughthe chemical treatment of a variety of ores.
Characteristics
Silver is somewhat rare and is considered a commercially precious metal with many uses.Pure silver is too soft and usually too expensive for many commercial uses, and thus it isalloyed with other metals, usually copper, making it not only stronger but also less expensive.The purity of silver is expressed in the term “fitness,” which describes the amount of silverin the item. Fitness is just a multiple of 10 times the silver content in an item. For instance,sterling silver should be 93% (or at least 92.5%) pure silver and 7% copper or some othermetal. The fitness rating for pure silver is 1000. Therefore, the rating for sterling silver is 930,and most sliver jewelry is rated at about 800. This is another way of saying that most silverjewelry is about 20% copper or other less valuable metal.
Many people are fooled when they buy Mexican or German silver jewelry, thinking theyare purchasing a semiprecious metal. These forms of “silver” jewelry go under many names,including Mexican silver, German silver, Afghan silver, Austrian silver, Brazilian silver, Nevadasilver, Sonara silver, Tyrol silver, Venetian silver, or just the name “silver” with quotes aroundit. None of these jewelry items, under these names or under any other names, contain anysilver. These metals are alloys of copper, nickel, and zinc.
History
Slag dumps in Asia Minor and on islands in the Aegean Sea indicate that man learned to separate silver from lead as early as 3000 B.C. Silver occurs native and in ores such as argentite (Ag2S) and horn silver (AgCl); lead, lead-zinc, copper, gold, and copper-nickel ores are principal sources. Mexico, Canada, Peru, and the U.S. are the principal silver producers in the western hemisphere. Silver is also recovered during electrolytic refining of copper. Commercial fine silver contains at least 99.9% silver. Purities of 99.999+% are available commercially. Pure silver has a brilliant white metallic luster. It is a little harder than gold and is very ductile and malleable, being exceeded only by gold and perhaps palladium. Pure silver has the highest electrical and thermal conductivity of all metals, and possesses the lowest contact resistance. It is stable in pure air and water, but tarnishes when exposed to ozone, hydrogen sulfide, or air containing sulfur. The alloys of silver are important. Sterling silver is used for jewelry, silverware, etc. where appearance is paramount. This alloy contains 92.5% silver, the remainder being copper or some other metal. Silver is of utmost importance in photography, about 30% of the U.S. industrial consumption going into this application. It is used for dental alloys. Silver is used in making solder and brazing alloys, electrical contacts, and high capacity silver–zinc and silver–cadmium batteries. Silver paints are used for making printed circuits. It is used in mirror production and may be deposited on glass or metals by chemical deposition, electrodeposition, or by evaporation. When freshly deposited, it is the best reflector of visible light known, but is rapidly tarnishes and loses much of its reflectance. It is a poor reflector of ultraviolet. Silver fulminate (Ag2C2N2O2), a powerful explosive, is sometimes formed during the silvering process. Silver iodide is used in seeding clouds to produce rain. Silver chloride has interesting optical properties as it can be made transparent; it also is a cement for glass. Silver nitrate, or lunar caustic, the most important silver compound, is used extensively in photography. While silver itself is not considered to be toxic, most of its salts are poisonous. Natural silver contains two stable isotopes. Fifty-six other radioactive isotopes and isomers are known. Silver compounds can be absorbed in the circulatory system and reduced silver deposited in the various tissues of the body. A condition, known as argyria, results with a greyish pigmentation of the skin and mucous membranes. Silver has germicidal effects and kills many lower organisms effectively without harm to higher animals. Silver for centuries has been used traditionally for coinage by many countries of the world. In recent times, however, consumption of silver has at times greatly exceeded the output. In 1939, the price of silver was fixed by the U.S. Treasury at 71¢/troy oz., and at 90.5¢/troy oz. in 1946. In November 1961 the U.S. Treasury suspended sales of nonmonetized silver, and the price stabilized for a time at about $1.29, the melt-down value of silver U.S. coins. The Coinage Act of 1965 authorized a change in the metallic composition of the three U.S. subsidiary denominations to clad or composite type coins. This was the first change in U.S. coinage since the monetary system was established in 1792. Clad dimes and quarters are made of an outer layer of 75% Cu and 25% Ni bonded to a central core of pure Cu. The composition of the oneand five-cent pieces remains unchanged. One-cent coins are 95% Cu and 5% Zn. Five-cent coins are 75% Cu and 25% Ni. Old silver dollars are 90% Ag and 10% Cu. Earlier subsidiary coins of 90% Ag and 10% Cu officially were to circulate alongside the clad coins; however, in practice they have largely disappeared (Gresham’s Law), as the value of the silver is now greater than their exchange value. Silver coins of other countries have largely been replaced with coins made of other metals. On June 24, 1968, the U.S. Government ceased to redeem U.S. Silver Certificates with silver. Since that time, the price of silver has fluctuated widely. As of January 2002, the price of silver was about $4.10/troy oz. (13¢/g); however the price has fluctuated considerably due to market instability. The price of silver in 2001 was only about four times the cost of the metal about 150 years ago. This has largely been caused by Central Banks disposing of some of their silver reserves and the development of more productive mines with better refining methods. Also, silver has been displaced by other metals or processes, such as digital photography.
The Uses of Silver
For coinage, most frequently alloyed with copper or gold; for manufacture of tableware, mirrors, jewelry, ornaments; for electroplating; for making vessels and apparatus used in manufacture of medicinal chemicals, in processing foods and beverages, in handling organic acids; as catalyst in hydrogenation and oxidation processes; as ingredient of dental alloys. Has been used for purification of drinking water because of toxicity to bacteria and lower forms of life. Some salts used in photography.
The Uses of Silver
This malleable white metal is found as argentite (Ag2S) and horn silver (AgCl) or in lead and copper ore. Copper plates coated with a thin layer of elemental silver and fumed with iodine were used by Niépce and Daguerre. Aside from the heliograph and physautotype, silver halide compounds were the basis of all photographic processes used in the camera and most of the printing processes during the 19th century.
The Uses of Silver
Silver has a multitude of uses and practical applications both in its elemental metallic formand as a part of its many compounds. Its excellent electrical conductivity makes it ideal for usein electronic products, such a computer components and high-quality electronic equipment.It would be an ideal metal for forming the wiring in homes and transmission lines, if it weremore abundant and less expensive.
Metallic silver has been used for centuries as a coinage metal in many countries. Theamount of silver now used to make coins in the United States has been reduced drastically byalloying other metals such as copper, zinc, and nickel with silver.
Silver is used as a catalyst to speed up chemical reactions, in water purification, and inspecial high-performance batteries (cells). Its high reflectivity makes it ideal as a reflectivecoating for mirrors.
Several of its compounds were not only useful but even essential for the predigital photographicindustry. Several of the silver salts, such as silver nitrate, silver bromide, and silverchloride, are sensitive to light and, thus, when mixed with a gel-type coating on photographicfilm or paper, can be used to form light images. Most of the silver used in the United Statesis used in photography.
Photochromic (transition) eyeglasses that darken as they are exposed to sunlight have asmall amount of silver chloride imbedded in the glass that forms a thin layer of metallic silverthat darkens the lens when struck by sunlight. This photosensitive chemical activity is thenreversed when the eyeglasses are removed from the light. This chemical reversal results from asmall amount of copper ions placed in the glass. This reaction is repeated each time the lensesare exposed to sunlight.
The Uses of Silver
Silver is a precious metal, used in jewelryand ornaments Other applications includeits use in photography, electroplating, dentalalloys, high-capacity batteries, printed circuits,coins, and mirrors. Silver is stable in air, and it is utilized in reflecting mirrors. The film vacuum evaporated on a quartz plate with the thickness of 2–55 nm shows the transmittance maximum at λ: 321.5 nm and works as a narrow band filter.
Definition
A transition metal that occurs native and as the sulfide (Ag2S) and chloride (AgCl). It is extracted as a by-product in refining copper and lead ores. Silver darkens in air due to the formation of silver sulfide. It is used in coinage alloys, tableware, and jewelry. Silver compounds are used in photography. Symbol: Ag; m.p. 961.93°C; b.p. 2212°C; r.d. 10.5 (20°C); p.n. 47; r.a.m. 107.8682.
Definition
silver: Symbol Ag. A white lustroussoft metallic transition element; a.n.47; r.a.m. 107.87; r.d. 10.5; m.p.961.93°C; b.p. 2212°C. It occurs as theelement and as the minerals argentite(Ag2S) and horn silver (AgCl). It isalso present in ores of lead and copper,and is extracted as a by-productof smelting and refining these metals.The element is used in jewellery,tableware, etc., and silver compoundsare used in photography.Chemically, silver is less reactivethan copper. A dark silver sulphideforms when silver tarnishes in air becauseof the presence of sulphurcompounds. Silver(I) ionic salts exist(e.g. AgNO3, AgCl) and there are anumber of silver(II) complexes.
Background
Silver (Ag) is a chemical element that belongs in the family of transition metals in the periodic table. It has a high electrical conductivity, thermal conductivity, and reflectivity. Silver exists as a pure elemental form, alloy with other metals, and mineral. Having critical roles in various applications inducing chemical and industrial fields, silver compounds have also been used in the field of medicine for centuries due to their broad-spectrum biological actions. Silver nanoparticles especially have been widely used in industrial, household, and healthcare-related products due to their potent antimicrobial activity. Silver nitrate and Silver sulfadiazine have been used as topical antibacterial agents for the treatment of skin infections, while Silver sulfadiazine has also been valued for topical burn treatment . Silver and its compounds have been used in trials studying the management of dental caries since the 1800s, and they may be found in dental pastes as an active ingredients. However, some drawbacks of dental use of silver compounds include tooth discolouration and pulp irritation .
Indications
Indicated for the treatment of acne for topical use or the management of dental caries for dental use.
General Description
Silver is a noble metal, extensively used in SERS, photocatalysis and solar cells. The surface of silver can be functionalized to attain specific properties such as biocompatibility and vapor selectivity of sensors.3 Iodized silver foils and thin films find potential use as SERS-active metal substrates.1 Cu substrates laminated with Ag foils, have compatible coefficient of thermal expansion (CTE), to be used for electronic packaging. Porous ZnO nanoplates deposited on silver foil with tunable hydrophobicity may be fabricated.3
Reactivity Profile
Silver reacts violently with chlorine trifluoride (in the presence of carbon) [Mellor 2 Supp. 1 1956]. Bromoazide explodes on contact with Silver foil. Acetylene forms an insoluble acetylide with Silver [Von Schwartz 1918 p. 142 ]. When Silver is treated with nitric acid in the presence of ethyl alcohol, Silver fulminate, which can detonated may be formed. Ethyleneimine forms explosive compounds with Silver, hence Silver solder should not be used to fabricate equipment for handling ethyleneimine. Finely divided Silver and strong solutions of hydrogen peroxide may explode [Mellor 1:936 1946-47)]. Incompatible with oxalic acid and tartaric acid [Nav Aer. 09-01-505 1956]. Silver can form explosive salts with azidrine. ("Ethyleneimine" Brocure 125-521-65, Midland (Mich.), Dow Chemical Co., 1965). Ammonia forms explosive compounds with gold, mercury, or Silver. (Eggeman, Tim. "Ammonia" Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2001.). Acetylene and ammonia can form explosive Silver salts in contact with Ag. (Renner, Hermann, Gunther Schlamp. “Silver, Silver Compounds, and Silver Alloys." Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA. 2001.)
Hazard
Toxic material.
Health Hazard
The acute toxicity of silver metal is low. The acute toxicity of soluble silver
compounds depends on the counterion and must be evaluated case by case. For
example, silver nitrate is strongly corrosive and can cause burns and permanent
damage to the eyes and skin.
Chronic exposure to silver or silver salts can cause a local or generalized darkening
of the mucous membranes, skin, and eyes known as argyria. The other chronic
effects of silver compounds must be evaluated individually.
Fire Hazard
Dust is flammable.
Flammability and Explosibility
Silver and most soluble silver compounds are not combustible. However, silver
nitrate and certain other silver compounds are oxidizers and can increase the
flammability of combustible materials.
Silver acetylide, azide, fulminate, oxalate mixtures, styphnate, tartarate mixtures,
and tetrazene are all explosives and must be handled as such.
Pharmaceutical Applications
The name silver is derived from the Saxon word ‘siloflur’, which has been subsequently transformed into
the German word ‘Silabar’ followed by ‘Silber’ and the English word ‘silver’. Romans called the element
‘argentum’, and this is where the symbol Ag derives from.
Silver is widely distributed in nature. It can be found in its native form and in various ores such as argentite
(Ag2S), which is the most important ore mineral for silver, and horn silver (AgCl). The principal sources of
silver are copper, copper–nickel, gold, lead and lead–zinc ores, which can be mainly found in Peru, Mexico,
China and Australia.
Silver has no known active biological role in the human body, and the levels of Ag+ within the body are
below detection limits. The metal has been used for thousands of years mainly as ornamental metal or for
coins.
Furthermore, silver has been used for medicinal purposes since 1000 BC. It was known that water would
keep fresh if it was kept in a silver pitcher; for example, Alexander the Great (356–323 BC) used to transport
his water supplies in silver pitchers during the Persian War. A piece of silver was also used, for example,
to keep milk fresh, before any household refrigeration was developed. In 1869, Ravelin proved that silver
in low doses acts as an antimicrobial. Around the same time, the Swiss botanist von N?geli showed that
already at very low concentration Ag+ can kill the green algae spirogyra in fresh water. This work inspired
the gynaecologist Crede to recommended use of AgNO3
drops on new born children with conjunctivitis.
Pharmacokinetics
Silver exhibits a broad-spectrum antimicrobial activity. Silver ions were shown to mediate an effective antibacterial action against Streptococcus mutans, one of major bacteria present in the human oral cavity and one of etiological microorganism of dental caries . A study reported a dose-dependent antimicrobial activity of silver nanoparticles against MRSA and non-MRSA bacteria . Silver nanoparticles were also shown to mediate antibacterial activity against Gram-positive S. aureus and Gram-negative E. coli by inhibiting the growth .
In experimental dinitrochlorobenzene-induced inflammatory models in porcine or murine skin, topical application of silver nitrate and nanocrystalline silver were shown to exert anti-inflammatory effects associated with lymphocyte apoptosis, decreased expression of pro-inflammatory cytokines, and reduced gelatinase activity . In a rat model of ulcerative colitis, orally or intracolonically administered nanocrystalline silver were shown to suppress matrix metalloproteinase (MMP-9), tumour necrosis factor (TNF), and interleukin-β (IL-β) and IL-12 .
Safety Profile
Human systemic effects by inhalation: skin effects. Inhalation of dusts can cause argyrosis. Questionable carcinogen with experimental tumorigenic data. Flammable in the form of dust when exposed to flame or by chemical reaction with C2H2, NH3, bromoazide, ClF3 ethyleneimine, H2O2, oxalic acid, H2SO4, tartaric acid. Incompatible with acetylene, acetylene compounds, aziridine, bromine azide, 3-bromopropyne, carboxylic acids, copper + ethylene glycol, electrolytes + zinc, ethanol + nitric acid, ethylene oxide, ethyl hydroperoxide, ethyleneimine, iodoform, nitric acid, ozonides, peroxomonosulfuric acid, peroxyformic acid. See also POWDERED METALS and SILVER COMPOUNDS.
Potential Exposure
Silver may be alloyed with copper, aluminum, cadmium, lead, or antimony. The alloys are used in the manufacture of silverware, jewelry, coins, ornaments, plates, commutators, scientific instruments; automobile bearing; and grids in storage batteries. Silver is used in chromenickel steels, in solders and brazing alloys; in the application of metallic films on glass and ceramics, to increase corrosion resistance to sulfuric acid, in photographic films, plates and paper; as an electroplated undercoating for nickel and chrome; as a bactericide for sterilizing water; fruit juices; vinegar, etc.; in bus bars and windings in electrical plants; in dental amalgams; and as a chemical catalyst in the synthesis of aldehydes. Because of its resistance to acetic and other food acids, it is utilized in the manufacture of pipes, valves, vats, pasteurizing coils and nozzles for the milk, vinegar, cider, brewing, and acetate rayon silk industries.
Carcinogenicity
The U.S. Department of Health
and Human Resources has extensively monitored published
studies on the occupational therapeutic and domestic exposures
to metals over many years, but has failed so far to
identify unequivocal clinical evidence that silver is carcinogenic
to humans under any circumstances. On the basis of
human experience and supportive studies in experimental
animals, silver is currently classified as a noncarcinogen
(62, 94, 141). On occasions patients exposed to silver in
antibiotic prostheses and other devices have died from
cancer, but in each case the role of silver in the etiology
of the malignancies was not proven.
It is expected that human contact with any of the radioactive
isotopes of silver may lead to local or other carcinogenic
changes in humans through the action of the radioactive
emissions as have been reported with gold in jewelry. No
cases have been seen to date.
Environmental Fate
Silver is a rare element, which occurs naturally in its pure form. It is a white, lustrous, relatively soft, and very malleable metal. Silver has an average abundance of about 0.1 ppm in the Earth’s crust and about 0.3 ppm in soils. It exists in four oxidation states (0,+1,+2,and +3). Silver occurs primarily as sulfides with iron, lead, tellurides, and with gold. Silver is found in surface waters as sulfide, bicarbonate, or sulfate salts, as part of complex ions with chlorides and sulfates and adsorbed onto particulate matter. Silver is released through natural processes, for example, erosion of soils. Sources of atmospheric contamination arise from processing of ores, steel refining, cement manufacture, fossil fuel combustion, and municipal waste incineration. Of anthropomorphic release, over 75% was estimated to be from disposal of solid waste. Ore smelting and fossil fuel combustion can emit fine particulates that may be transported long distances and deposited with precipitation. The major source of release to surface waters is effluent from photographic processing. Releases from the photographic industry and from disposal of sewage sludge and refuse are the major sources of soil contamination with silver. Silver can leach into groundwater, which can be extenuated in acidic conditions. Silver can bioconcentrate in fish and invertebrates.
Metabolism
No pharmacokinetic data on metabolic pathways.
Storage
Most silver compounds should be protected from light during storage or while in use.
Purification Methods
For purification by electrolysis, see Craig et al. [J Res Nat Bur Stand 64A 381 1960]. For purification of crude, or silver residues to pure silver see Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 1028-1030 1963, and for the preparation of colloidal silver see ibid (Ed. Brauer) p 1034.
Toxicity evaluation
Ag+ is the biologically active form. Silver is not an essential mineral supplement and has no known physiologic function. While specific mechanisms of toxicity are unclear, silver has high affinity for sulfhydryl groups and proteins. The deposition of silver in tissues is the result of precipitation of insoluble silver salts, such as silver chloride and silver phosphate. These insoluble salts appear to be transformed into soluble silver sulfide albuminates; to form complexes with amino or carboxyl groups in RNA, DNA, and proteins; or to be reduced to metallic silver by ascorbic acid or catecholamines. These could lead to alteration of a number of cellular processes.
Incompatibilities
Dust may form explosive mixture with air. Powders are incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides May react and/or form dangerous or explosive compounds, with acetylene, ammonia, halogens, hydrogen peroxide; bromoazide, concentrated or strong acids, oxalic acid, tartaric acid, chlorine trifluoride, ethyleneimine.
Waste Disposal
Recovery, wherever possible, in view of economic value of silver. Techniques for silver recovery from photoprocessing and electroplating wastewaters have been developed and patented.
Properties of Silver
| Melting point: | 960 °C(lit.) |
| Boiling point: | 2212 °C(lit.) |
| Density | 1.135 g/mL at 25 °C |
| vapor density | 5.8 (vs air) |
| vapor pressure | 0.05 ( 20 °C) |
| refractive index | n |
| Flash point: | 232 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | wool |
| color | Yellow |
| Specific Gravity | 10.49 |
| Odor | Odorless |
| Resistivity | 1-3 * 10^-5 Ω-cm (conductive paste) &_& 1.59 μΩ-cm, 20°C |
| Water Solubility | insoluble |
| Sensitive | Light Sensitive |
| Merck | 13,8577 |
| Exposure limits | TLV-TWA (metal dusts and fumes) 0.1
mg/m3 (ACGIH), 0.01 mg/m3 (MSHA and
OSHA), soluble compounds 0.01 mg/m3
(AIGIH). |
| Stability: | Stable. Substances to be avoided include strong acids and strong bases, tartaric acid, oxalic acid. Blackened by contact with ozone, hydrogen sulfide, sulfur. Powder is highly flammable. |
| CAS DataBase Reference | 7440-22-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Silver(7440-22-4) |
| EPA Substance Registry System | Silver (7440-22-4) |
Safety information for Silver
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Silver
Silver manufacturer
A.B. Enterprises
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Silver 99%View Details
Silver 99%View Details -
 Silver plating solution, Metal content ≈28.7g/L CASView Details
Silver plating solution, Metal content ≈28.7g/L CASView Details -
 Silver plating solution, Metal content ≈28.7g/L CASView Details
Silver plating solution, Metal content ≈28.7g/L CASView Details -
 Silver CASView Details
Silver CASView Details -
 Silver CASView Details
Silver CASView Details -
 Silver CASView Details
Silver CASView Details -
 Silver CASView Details
Silver CASView Details -
 Silver, dispersion CASView Details
Silver, dispersion CASView Details