YTTERBIUM
Synonym(s):;Ytterbium
- CAS NO.:7440-64-4
- Empirical Formula: Yb
- Molecular Weight: 173.04
- MDL number: MFCD00011286
- EINECS: 231-173-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
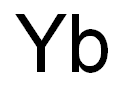
What is YTTERBIUM?
Chemical properties
metal chips
Physical properties
Ytterbium is a silvery, soft, malleable, and ductile metal with a lustrous metallic shine.It is slightly reactive in air or water at room temperatures. Ytterbium is located next to lastof the rare-earths in the lanthanide series. It slowly oxidizes as it reacts with oxygen in theatmosphere, forming a somewhat duller coating. Ytterbium was the first rare-earth to bediscovered by Carl Gustof Mosander in 1843. More of it exists in the Earth’s crust than oncewas believed.

It was often confused with other rare-earths and was known by two other names, aldebaraniumand cassiopeium. Ytterbium’s melting point is 819°C, its boiling point is 1,196°C, andits density is 6.9654g/cm3.
Isotopes
There are a total of 37 isotopes of ytterbium. Seven of these are stable, andthey make up all of the natural ytterbium found on Earth. One of these isotopes (Yb-176) has such a long half-life (1.6×10+17years) that it contributes 12.76% of the naturalytterbium existing on Earth, and thus it is considered stable. All the other 30 isotopes areartificially radioactive and produced by nuclear fission in nuclear reactors with half-livesranging from a fraction of a second to 32 days.
Origin of Name
Ytterbium is named for the Ytterby quarry located in Sweden.
Occurrence
Ytterbium is the 45th most abundant element, and it ranks 10th in abundance (2.7 ppm)among the 17 rare-earths found in the Earth’s crust.
It is found in ores along with other rare-earths that were first found in the Ytterby quarryof Sweden. These ores are xenotime, euxenite, gadolinite, and monazite. Monazite river sand is the main source of ytterbium, which is found in India and Brazil and the beaches of Florida.Ytterbium is also found as a decay product of the fission reaction in nuclear reactors.
History
Marignac in 1878 discovered a new component, which he called ytterbia, in the Earth then known as erbia. In 1907, Urbain separated ytterbia into two components, which he called neoytterbia and lutecia. The elements in these earths are now known as ytterbium and lutetium, respectively. These elements are identical with aldebaranium and cassiopeium, discovered independently and at about the same time by von Welsbach. Ytterbium occurs along with other rare earths in a number of rare minerals. It is commercially recovered principally from monazite sand, which contains about 0.03%. Ion-exchange and solvent extraction techniques developed in recent years have greatly simplified the separation of the rare earths from one another. The element was first prepared by Klemm and Bonner in 1937 by reducing ytterbium trichloride with potassium. Their metal was mixed, however, with KCl. Daane, Dennison, and Spedding prepared a much purer form in 1953 from which the chemical and physical properties of the element could be determined. Ytterbium has a bright silvery luster, is soft, malleable, and quite ductile. While the element is fairly stable, it should be kept in closed containers to protect it from air and moisture. Ytterbium is readily attacked and dissolved by dilute and concentrated mineral acids and reacts slowly with water. Ytterbium has three allotropic forms with transformation points at –13° and 795°C. The beta form is a room-temperature, face-centered, cubic modification, while the high-temperature gamma form is a body-centered cubic form. Another bodycentered cubic phase has recently been found to be stable at high pressures at room temperatures. The beta form ordinarily has metallic-type conductivity, but becomes a semiconductor when the pressure is increased above 16,000 atm. The electrical resistance increases tenfold as the pressure is increased to 39,000 atm and drops to about 80% of its standard temperature- pressure resistivity at a pressure of 40,000 atm. Natural ytterbium is a mixture of seven stable isotopes. Twenty-six other unstable isotopes and isomers are known. Ytterbium metal has possible use in improving the grain refinement, strength, and other mechanical properties of stainless steel. One isotope is reported to have been used as a radiation source as a substitute for a portable X-ray machine where electricity is unavailable. Few other uses have been found. Ytterbium metal is available with a purity of about 99.9% for about $10/g. Ytterbium has a low acute toxicity rating.
Characteristics
In the past there was some confusion about the rare-earths because they are not really earthsat all, but rather binary compounds of oxides of metals. Compounding the confusion was thefact that they were always found combined with several other rare-earths.
The salts of ytterbium are paramagnetic, which exhibit weaker magnetic fields than do ironmagnets.
The Uses of YTTERBIUM
Ytterbium is being applied to numerous fiber amplifier and fiber optic technologies and in various lasing applications.
Ytterbium metal increases its electrical resistance when subjected to very high stresses. This property is used in stress gauges for monitoring ground deformations from earthquakes and nuclear explosions.
Ytterbium can also be used as a dopant to help improve the grain refinement, strength, and other mechanical properties of stainless steel. Some Ytterbium alloys have rarely been used in dentistry.
It is also used as in thermal barrier system bond coatings on nickel, iron and other transitional metal alloy substrates.
Ytterbium Metal, is being applied in improving the grain refinement, strength, and other mechanical properties of stainless steel and alloys. The 169Yb has been used as a radiation source in portable X-ray machines.169Yb is also used in nuclear medicine. Ytterbium can also be used as a dopant to help improve the grain refinement, strength, and other mechanical properties of stainless steel. Some ytterbium alloys have rarely been used in dentistry.
Ytterbium Metal can be further processed to various shapes of ingots, pieces, wires, foils, slabs, rods, discs and powder.
The Uses of YTTERBIUM
There is not much commercial use for ytterbium. Radioactive ytterbium can be used for asmall portable X-ray source and as an alloy to make special types of strong steel. The oxides ofytterbium are used to make lasers and some synthetic gemstones.
The Uses of YTTERBIUM
Used for phosphors, ceramic capacitors, ferrite devices, pulsed lasers and catalysts. Use in improving the strength and mechanical properties of stainless steel. Used in Physical Vapor Deposition (PVD) processes, including thermal and electron-beam (e-beam) evaporation, for the preparation of thin films.
Definition
A metallic element. A rare-earth metal of yttrium subgroup, atomic number 70, aw 173.04, valence of 2, 3; exists in α and β forms, the latter being semiconductive at pressures above 16,000 atm. There are seven natural isotopes.
Definition
A soft malleable silvery element having two allotropes and belonging to the lanthanoid series of metals. It occurs in association with other lanthanoids. Ytterbium has been used to improve the mechanical properties of steel. Symbol: Yb; m.p. 824°C; b.p. 1193°C; r.d. 6.965 (20°C); p.n. 70; r.a.m. 173.04.
Definition
ytterbium: Symbol Yb. A silverymetallic element belonging to thelanthanoids; a.n. 70; r.a.m. 173.04;r.d. 6.965 (20°C); m.p. 819°C; b.p.1194°C. It occurs in gadolinite, monazite,and xenotime. There are sevennatural isotopes and ten artificial isotopesare known. It is used in certainsteels. The element was discoveredby Jean de Marignac (1817–94) in1878.
Hazard
Ytterbium dust and powder can explode and may be toxic if inhaled. The compound.ytterbium arsenate is a poison.
Safety Profile
As a lanthanon it maj7 have an anticoagulant action on blood. Questionable carcinogen with experimental tumorigenic data. Flammable in the form of dust when reacted with air, halogens. See also LANTHANUM and RARE EARTHS.
Properties of YTTERBIUM
| Melting point: | 824 °C(lit.) |
| Boiling point: | 1196 °C(lit.) |
| Density | 6.54 g/mL at 25 °C(lit.) |
| storage temp. | Flammables area |
| solubility | soluble in dilute acid solutions |
| form | powder |
| color | Silver-gray |
| Specific Gravity | 6.97 |
| Resistivity | 28 μΩ-cm, 20°C |
| Water Solubility | It reacts slowly with water and is soluble in dilute acids. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,10160 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7440-64-4(CAS DataBase Reference) |
| EPA Substance Registry System | Ytterbium (7440-64-4) |
Safety information for YTTERBIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for YTTERBIUM
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




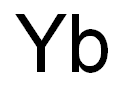
You may like
-
 Ytterbium, May contain up to 0.15% Calcium CAS 7440-64-4View Details
Ytterbium, May contain up to 0.15% Calcium CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium, May contain up to 0.15% Calcium CAS 7440-64-4View Details
Ytterbium, May contain up to 0.15% Calcium CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium foil, 0.3mm (0.01 in.) thick CAS 7440-64-4View Details
Ytterbium foil, 0.3mm (0.01 in.) thick CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium foil, 0.3mm (0.01 in.) thick CAS 7440-64-4View Details
Ytterbium foil, 0.3mm (0.01 in.) thick CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium foil, 1.0mm (0.04 in.) thick CAS 7440-64-4View Details
Ytterbium foil, 1.0mm (0.04 in.) thick CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium foil, 1.0mm (0.04 in.) thick CAS 7440-64-4View Details
Ytterbium foil, 1.0mm (0.04 in.) thick CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium ingot CAS 7440-64-4View Details
Ytterbium ingot CAS 7440-64-4View Details
7440-64-4 -
 Ytterbium CAS 7440-64-4View Details
Ytterbium CAS 7440-64-4View Details
7440-64-4