sec-Butyl chloroformate
- CAS NO.:17462-58-7
- Empirical Formula: C5H9ClO2
- Molecular Weight: 136.58
- MDL number: MFCD00043796
- EINECS: 241-475-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
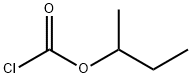
What is sec-Butyl chloroformate?
The Uses of sec-Butyl chloroformate
sec-Butyl Chloroformate-D3 is an intermediate in the synthesis of Picaridin-d3(P436002) which acts on certain olfactory receptor cell types to reduce the activating or attracting effect of odor sources.Insect repellent.
General Description
A colorless liquid. Insoluble in water and more dense than water. Contact may severely irritate skin, eyes, and mucous membranes. Very toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Reacts exothermically with moisture in air to generate fumes of hydrochloric acid. Decomposes slowly in water. Insoluble in water.
Reactivity Profile
sec-Butyl chloroformate reacts with water to form sec-butanol, HCl, and CO2. Attacks many metals, especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Properties of sec-Butyl chloroformate
| Boiling point: | 126 °C |
| Density | 1.081±0.06 g/cm3(Predicted) |
| EPA Substance Registry System | sec-Butyl chloroformate (17462-58-7) |
Safety information for sec-Butyl chloroformate
Computed Descriptors for sec-Butyl chloroformate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran


You may like
-
 17462-58-7 sec-butyl chloroformate 98%View Details
17462-58-7 sec-butyl chloroformate 98%View Details
17462-58-7 -
 Sec-butyl chloroformate 95% CAS 17462-58-7View Details
Sec-butyl chloroformate 95% CAS 17462-58-7View Details
17462-58-7 -
 sec-Butyl chloroformate 95% (GC) CAS 17462-58-7View Details
sec-Butyl chloroformate 95% (GC) CAS 17462-58-7View Details
17462-58-7 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0