butyl rubber
- CAS NO.:9010-85-9
- Empirical Formula: C9H16
- Molecular Weight: 124.23
- MDL number: MFCD00217721
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
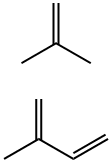
What is butyl rubber?
Description
A synthetic copolymer containing from 0.5 to 2.0 molar percent of isoprene, the remainder, respectively, consisting of isobutylene. It is prepared by copolymerization of isobutylene and isoprene in methyl chloride solution, using aluminum chloride as the catalyst. After completion of polymerization, the rubber particles aretreated with hot water containing a suitable food-grade deagglomerating agent, such as stearic acid. Finally, the coagulum is dried to remove residual volatiles.
Chemical properties
Viscosity controlling agent.
Chemical properties
Butyl rubber has the chemical resistance characteristic of saturated hydrocarbons. Oxidative degradation is slow, and the rubber may be further protected by incorporating antioxidants. Isobutylene is the major component of bulk rubber. It provides good aging resistance and low gas permeability. Isoprene, a minor component, enhances vulcanizability.
The Uses of butyl rubber
Butyl rubber is formed from the polymerization of isobutene (isobutylene) with small amounts of chloroprene, isoprene, or butadiene and may be halogenated. The reaction is carried out in a closed system with an aluminum chloride catalyst. Butyl rubber is highly impervious to gases, which makes it the rubber best suited for inner tubes and tires, air chambers, adhesives, and dielectrics. Isobutene is an anesthetic and asphyxiant gas and can be hazardous in concentrations high enough to produce asphyxia. At these concentrations, explosion is an additional hazard.

Production Methods
The bulk of butyl rubber is made by a slurry process using aluminum chloride at 98–99°C and methyl chloride as a diluent. The extremely rapid reaction is unique, and proceeds via cationic polymerization to completion at 100°C in less than a second. Butyl rubber may be vulcanized by three basic methods: accelerated sulfur vulcanization, cross-linking with dioxime and related dinitroso compounds, and polymethylol– phenol resin cure. Halogenated butyl rubber allows broadened vulcanization latitude and rate, and enhanced covulcanization to general-purpose elastomers while it maintains the unique attributes of the basic butyl molecule.
Preparation
Commercial butyl rubbers generally contain 1-3 mole % isoprene. In a
typical process, a solution of the monomers in methyl chloride is pre-cooled
to -lOO??C and then fed continuously into the base of a reactor together with
a solution of aluminium chloride (initiator) in the same diluent. The reaction
is highly exothermic and in order to maintain the temperature at -100??C the
reactor is fitted with a powerful stirrer and cooling coils containing liquid
ethylene. The polymer forms as a granular slurry which is displaced continuously from the top of the reactor. The slurry passes into a flash tank
where it is vigorously agitated with hot water. This treatment deactivates the
initiator and extracts water-soluble products. At the same time the diluent
and unreacted monomers are flashed off and are purified and re-used. Also at
this point a lubricant and an antioxidant are introduced into the polymer.
The lubricant, usually zinc stearate, prevents agglomeration of wet crumb
and the antioxidant, usually phenyl-p-naphthylamine, reduces degradation
during drying operations. The slurry then passes to a screen where wet
polymer crumb is isolated. The crumb is dried at about 95-170??C, fed
through an extruder and then hot milled. The object of these last operations
is to compact the polymer and remove all traces of residual water.
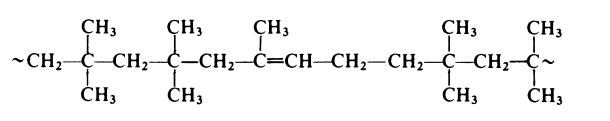
In butyl rubber the isoprene is present as random l,4-units and the
copolymer thus has the following form:
Definition
ChEBI: Isobutylene-isoprene copolymer is a hydrocarbon.
Industrial uses
Butyl rubbers have outstanding impermeabilityto gases and excellent oxidation andozone resistance. The chemical inertness is furtherreflected in lack of molecular-weightbreakdown during processing, thus permittingthe use of hot-mixing techniques for betterpolymer/filler interaction.
Advantages
Butyl rubber is chemically resistant to non-oxidizing dilute mineral acids, salts and alkalis, and a good chemical resistance to concentrated acids, except sulfuric and nitric acids. Moreover, it has a low permeability to air and an excellent resistance to aging and ozone. However, it is readily attacked by oxidizing chemicals, oils, benzene, and ketones and as a general rule it has also poor chemical resistance to petroleum and its derivatives and many organic chemicals. Moreover, butyl rubbers are sensitive to UV-irradiation (e.g., sunlight exposure). As with other rubbers, its mechanical properties can be largely improved by the vulcanization process.
Properties of butyl rubber
| EPA Substance Registry System | 1,3-Butadiene, 2-methyl-, polymer with 2-methyl-1-propene (9010-85-9) |
Safety information for butyl rubber
Computed Descriptors for butyl rubber
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 9010-85-9 Butyl rubber 99%View Details
9010-85-9 Butyl rubber 99%View Details
9010-85-9 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
