Butylparaben
Synonym(s):p-Hydroxybenzoic acid n-butyl ester;4-Hydroxybenzoic acid butyl ester;Butyl paraben;SPF
- CAS NO.:94-26-8
- Empirical Formula: C11H14O3
- Molecular Weight: 194.23
- MDL number: MFCD00016478
- EINECS: 202-318-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:52
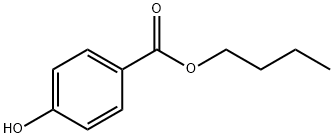
What is Butylparaben?
Description
Butylparaben is an antimicrobial agent used in pharmaceutical suspensions. It is act by inhibiting DNA, RNA, and enzymes (eg, ATPase and phosphotransferase) synthesis. Butylparaben may be used alone or with other parabens, chiefly methylparaben and/or propylparaben, in medications. It is common in many liquid and solid (gel cap) OTC products such as Tylenol, Drixoral, Maalox, and Mylanta. Unfortunately, butylparaben concentrations were seldom identified for OTC or prescription products. No attempt was made to identify butylparaben-containing dietary supplements.
Chemical properties
Butylparaben occurs as colorless crystals or a white, crystalline, odorless or almost odorless, tasteless powder.
The Uses of Butylparaben
preservative in many creams, lotions, ointments and other cosmetics, foods (salad dressings, mayonnaise, spiced sauces, mustard, frozen dairy products, baked products), pharmaceutical preparations and dentifrices. It is active against molds, fungi and yeasts, but less active against bacteria. ICU.
What are the applications of Application
Butyl Paraben is antimicrobial agent used in cosmetic products
Production Methods
Butylparaben is prepared by esterification of p-hydroxybenzoic acid with n-butanol.
Preparation
Butyl paraben is prepared by esterifying p-hydroxybenzoic acid with butyl alcohol in the presence of an acid catalyst, such as sulfuric acid, and an excess of the specific alcohol.
Definition
ChEBI: Butylparaben is an organic molecular entity. It is a Standardized Chemical Allergen. The physiologic effect of butylparaben is by means of Increased Histamine Release, and Cell-mediated Immunity.
General Description
Odorless white crystals or crystalline powder. Tasteless, but numbs the tongue. Aqueous solutions slightly acidic to litmus.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Butylparaben is incompatible with strong oxidizing agents and strong caustics.
Fire Hazard
Flash point data for Butylparaben are not available; however, Butylparaben is probably combustible.
Pharmaceutical Applications
Butylparaben is widely used as an antimicrobial preservative in
cosmetics and pharmaceutical formulations.
It may be used either alone or in combination with other paraben
esters or with other antimicrobial agents. In cosmetics, it is the
fourth most frequently used preservative.
As a group, the parabens are effective over a wide pH range and
have a broad spectrum of antimicrobial activity, although they are
most effective against yeasts and molds.
Owing to the poor solubility of the parabens, paraben salts,
particularly the sodium salt, are frequently used in formulations.
However, this may raise the pH of poorly buffered formulations.
See Methylparaben for further information.
Contact allergens
This substance is one of the parabens family. Parabens are esters formed by p-hydroxybenzoic acid and an alcohol. They are largely used as biocides in cosmetics and toiletries, medicaments, or food. They have synergistic power with other biocides. Parabens can induce allergic contact dermatitis, mainly in chronic dermatitis and wounded skin.
Safety
Butylparaben and other parabens are widely used as antimicrobial
preservatives in cosmetics and oral and topical pharmaceutical
formulations.
Systemically, no adverse reactions to parabens have been
reported, although they have been associated with hypersensitivity
reactions generally appearing as contact dematitis. Immediate
reactions with urticaria and bronchospasm have occurred rarely.
See Methylparaben for further information.
LD50 (mouse, IP): 0.23 g/kg
LD50 (mouse, oral): 13.2 g/kg
Metabolism
Not Available
Storage
Aqueous butylparaben solutions at pH 3–6 can be sterilized by autoclaving, without decomposition. At pH 3–6, aqueous solutions are stable (less than 10% decomposition) for up to about 4 years at room temperature, while solutions at pH 8 or above are subject to rapid hydrolysis (10% or more after about 60 days at room temperature).
Incompatibilities
The antimicrobial activity of butylparaben is considerably reduced
in the presence of nonionic surfactants as a result of micellization.
Absorption of butylparaben by plastics has not been reported but
appears probable given the behavior of other parabens. Some
pigments, e.g. ultramarine blue and yellow iron oxide, absorb
butylparaben and thus reduce its preservative properties.
Butylparaben is discolored in the presence of iron and is subject
to hydrolysis by weak alkalis and strong acids.
Regulatory Status
Butylparaben is regulated by the U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). In 1998 its pesticide registration status was listed as "cancelled" (U.S. EPA, 2003).
Included in the FDA Inactive Ingredients Database (injections; oral capsules, solutions, suspensions, syrups and tablets; rectal, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Properties of Butylparaben
| Melting point: | 67-70 °C(lit.) |
| Boiling point: | 156-157 °C3.5 mm Hg(lit.) |
| Density | 1.28 |
| vapor pressure | 0.002-0.113Pa at 20-50℃ |
| FEMA | 2203 | BUTYL P-HYDROXY BENZOATE |
| refractive index | 1.5115 (estimate) |
| Flash point: | 181℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO, ethyl acetate, methanol. |
| form | Crystalline Powder |
| pka | pKa 8.5 (Uncertain) |
| color | White to almost white |
| Odor | very faint phenolic |
| Water Solubility | <0.1 g/100 mL at 17 ºC |
| Merck | 14,1584 |
| JECFA Number | 870 |
| BRN | 1103741 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong alkalies. |
| CAS DataBase Reference | 94-26-8(CAS DataBase Reference) |
| NIST Chemistry Reference | P-hydroxybenzoic acid, n-butyl ester(94-26-8) |
| EPA Substance Registry System | Butylparaben (94-26-8) |
Safety information for Butylparaben
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Butylparaben
| InChIKey | QFOHBWFCKVYLES-UHFFFAOYSA-N |
Butylparaben manufacturer
Akhil Healthcare Private Limited
Bazayan & Co.
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Butyl paraben CAS 94-26-8View Details
Butyl paraben CAS 94-26-8View Details
94-26-8 -
![Butyl 4-Hydroxybenzoate [for Biochemical Research] CAS 94-26-8](https://img.chemicalbook.in//Content/image/CP5.jpg) Butyl 4-Hydroxybenzoate [for Biochemical Research] CAS 94-26-8View Details
Butyl 4-Hydroxybenzoate [for Biochemical Research] CAS 94-26-8View Details
94-26-8 -
 Butyl 4-Hydroxybenzoate CAS 94-26-8View Details
Butyl 4-Hydroxybenzoate CAS 94-26-8View Details
94-26-8 -
 Butyl 4-hydroxybenzoate 98% CAS 94-26-8View Details
Butyl 4-hydroxybenzoate 98% CAS 94-26-8View Details
94-26-8 -
 White Butyl-4-Hydroxybenzoate CAS: 94-26-8View Details
White Butyl-4-Hydroxybenzoate CAS: 94-26-8View Details
94-26-8 -
 Powder Butyl Paraben, 25 Kg, Packaging Type: Packets, BagView Details
Powder Butyl Paraben, 25 Kg, Packaging Type: Packets, BagView Details
94-26-8 -
 BUTYL PARABEN 99%, For Lab UseView Details
BUTYL PARABEN 99%, For Lab UseView Details
94-26-8 -
 Powder Butyl Paraben Cas No 94-26-8, For Industrial, Purity: 98%View Details
Powder Butyl Paraben Cas No 94-26-8, For Industrial, Purity: 98%View Details
94-26-8
