SB 225002
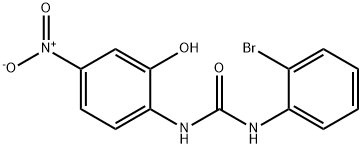
Synonym(s):N-(2-Hydroxy-4-nitrophenyl)-Nʹ-(2-bromophenyl)urea, CXCR2 Antagonist II;N-(2-Hydroxy-4-nitrophenyl)-N′-(2-bromophenyl)urea;SB 225002 - CAS 182498-32-4 - Calbiochem;SB-225002
- CAS NO.:182498-32-4
- Empirical Formula: C13H10BrN3O4
- Molecular Weight: 352.14
- MDL number: MFCD00954637
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is SB 225002?
Description
CXCR2 (IL-
The Uses of SB 225002
SB 225002 is a potent and selective CXCR2 antagonist with the ability to inhibit interleukin IL-8 binding to CXCR2.
What are the applications of Application
SB 225002 is a selective antagonist of CXCR2
Definition
ChEBI: 1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea is a nitrophenol.
Biological Activity
Potent and selective CXCR2 chemokine receptor antagonist (IC 50 = 22 nM) that displays > 150-fold selectivity over CXCR1 receptors. Causes inhibition of IL-8 and GRO α -mediated calcium mobilization in HL60 cells (IC 50 values are 8 and 10 nM respectively). Prevents IL-8-induced neutrophil chemotaxis in vitro and sequestration in vivo . Inhibits HIV replication in lymphocytes and macrophages.
Biochem/physiol Actions
SB-225002 is a potent nonpeptide inhibitor of chemokine receptor CXCR2 with an IC50 of 22 nM for inhibiting interleukin IL-8 binding to CXCR2 and > 150-fold selectivity over CXCR1 receptors. CXCR2 binds many different immune cell chemoattractants. SB-225002 is crucial for cancer progression and is involved in inflammatory diseases like COPD, rheumatoid arthritis, and ulcerative colitis.
storage
Store at RT
References
1) White et al. (1998), Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration; J. Biol. Chem. 273 10095 2) Du et al. (2013) SB225002 Promotes Mitotic Catastrophe in Chemo-Sensitive and -Resistant Ovarian Cancer Cells Independent of p53 Status In Vitro; PLoS One. 8 e54572 3) Shen et al. (2013), Interleukin-8 prevents oxidative stress-induced human endothelial cell senescence via telomerase activation; Int. Immunopharmacol. 16 261 4) Lane et al. (2001), Interleukin-8 Stimulates Human Immunodeficiency Virus Type 1 Replication and Is a Potential New target for Antiretroviral Therapy; J. Virol. 75 8195 5) Wu et al. (2015), CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation; J. Neuroinflammation 12 98
Properties of SB 225002
| Boiling point: | 401.6±45.0 °C(Predicted) |
| Density | 1.793±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble to 100mM in DMSO and to 50mM in ethanol |
| form | Yellow solid |
| pka | 5.29±0.10(Predicted) |
| color | white to beige |
| Sensitive | Moisture Sensitive |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 182498-32-4 |
Safety information for SB 225002
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for SB 225002
| InChIKey | MQBZVUNNWUIPMK-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![2-(5-Benzo[1,3]dioxol-5-yl-2-tert-butyl-3H-imidazol-4-yl)-6-methylpyridine hydrate hydrochloride](https://img.chemicalbook.in/CAS/GIF/694433-59-5.gif)
You may like
-
 Sb 225002 95% CAS 182498-32-4View Details
Sb 225002 95% CAS 182498-32-4View Details
182498-32-4 -
 SB 225002 CAS 182498-32-4View Details
SB 225002 CAS 182498-32-4View Details
182498-32-4 -
 SB 225002 CAS 182498-32-4View Details
SB 225002 CAS 182498-32-4View Details
182498-32-4 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4