Salicylic acid
Synonym(s):2-Hydroxybenzoic acid;Acidum salicylicum;Salicylic Acid
- CAS NO.:69-72-7
- Empirical Formula: C7H6O3
- Molecular Weight: 138.12
- MDL number: MFCD00002439
- EINECS: 200-712-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
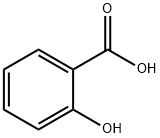
What is Salicylic acid?
Toxicity
Oral rat LD50: 891 mg/kg. Inhalation rat LC50: > 900 mg/m3/1hr. Irritation: skin rabbit: 500 mg/24H mild. Eye rabbit: 100 mg severe. Investigated a mutagen and reproductive effector.
Description
Salicylic acid (2-hydroxybenzoic acid) is a white solid first isolated from the bark of willow trees (Salix spp.), from which it gets its name. It also occurs as the free acid or its esters in many plant species.
In an early (1966) biosynthetic process, researchers at Kerr-McGee Oil Industries (now part of Andarko Petroleum) prepared salicylic acid via the microbial degradation of naphthalene. It is now commercially biosynthesized from phenylalanine.
Acetylsalicylic acid (aspirin), a prodrug to salicylic acid, is made by an entirely different process. Curiously, salicylic acid is also a metabolite of aspirin.
Salicylic acid and its esters are used as food preservatives, in skin-care products and other cosmetics, and in topical medicines. In 2015, J. L. Dangl, S. L. Lebeis, and co-workers at the University of North Carolina, Chapel Hill, discovered that native salicylic acid plays a role in determining which microbes are in the root microbiome of Arabidopsis thaliana, a weed that grows in Europe and Asia.
Physical properties
Salicylic acid. Appearance: white crystalline powder. Solubility: Absolutely soluble
in ethanol, soluble in ether and chloroform, slightly soluble in water and anhydrous
ether. Stability: Stable at room temperature, discomposes into phenol and carbon
dioxide after rapidly heated. It’s partially acidic.
Aspirin. Appearance: white crystal and decomposes at 136–140? °C. Melting
point: 136?°C.?Aspirin is the acetyl derivative of salicylic acid with weak acidity. Its
acidity coefficient is 3.5 at 25?°C. Stability: Aspirin decomposes rapidly in ammonium acetate, alkali metal of acetate, carbonate, citrate or hydroxide solutions.
There are two crystal forms of aspirin including crystal form I and II.
Occurrence
Unripe fruits and vegetables are natural sources of salicylic acid, particularly blackberries, blueberries, cantaloupes, dates, raisins, kiwi fruits, guavas, apricots, green pepper, olives, tomatoes, radish and chicory; also mushrooms. Some herbs and spices contain quite high amounts, although meat, poultry, fish, eggs and dairy products all have little to no salicylates. Of the legumes, seeds, nuts, and cereals, only almonds, water chestnuts and peanuts have significant amounts.
History
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers . This remedy was also mentioned in texts from ancient Sumer , Lebanon , and As syria .
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
The Uses of Salicylic acid
Salicylic acid is a beta-hydroxy acid with keratolytic and anti-inflammatory properties. It helps dissolve the top layer of keratinocytes, improving the appearance and feel of the skin. It's an active ingredient in acne-fighting products and is widely used in anti-acne soaps and lotions. In medicine, it's used to treat and prevent acne and other skin conditions such as warts, psoriasis, calluses, and corns. It's also used as a food preservative, antiseptic, and disinfectant. It also has pain-relieving and fever-reducing properties.
Indications
Key additive in many skin-care products for the treatment of acne, psoriasis, callouses, corns, keratosis pilaris and warts.
Background
A compound obtained from the bark of the white willow and wintergreen leaves, and also prepared synthetically. It has bacteriostatic, fungicidal, and keratolytic actions. Its salts, the salicylates, are used as analgesics.
Definition
A crystalline aromatic carboxylic acid. It is used in medicines, as an antiseptic, and in the manufacture of azo dyes. Its ethanoyl (acetyl) ester is aspirin. See aspirin; methyl salicylate.
Production Methods
Salicylic acid may be obtained (1) from oil of wintergreen, which contains methyl salicylate, or (2) by heating dry sodium phenate C6H5ONa plus carbon dioxide under pressure at 130 °C (266 °F) and recovering from the resulting sodium salicylate by adding dilute sulfuric acid.
Indications
Salicylic acid is indicated for the treatment of superficial fungal infections, acne, psoriasis, seborrheic dermatitis, warts, and other scaly skin conditions.
brand name
Advanced Pain Relief Callus Removers (Schering-Plough HealthCare); Advanced Pain Relief Corn Removers (Schering-Plough HealthCare); Clear Away Wart Remover (Schering-Plough HealthCare); Compound W (Whitehall-Robins); Dr. Scholl’s Callus Removers (Schering-Plough HealthCare); Dr. Scholl’s Corn Removers (Schering-Plough HealthCare); Dr. Scholl’s Wart Remover Kit (Schering-Plough HealthCare); Duofilm Wart Remover (Schering-Plough HealthCare); Duoplant (Stiefel); Freezone (Whitehall-Robins); Ionil (Galderma); Ionil-Plus (Galderma); Salicylic Acid Soap (Stiefel); Saligel (Stiefel); Stri-Dex (Sterling Health U.S.A.).
Synthesis Reference(s)
The Journal of Organic Chemistry, 27, p. 3551, 1962 DOI: 10.1021/jo01057a035
Tetrahedron Letters, 37, p. 153, 1996 DOI: 10.1016/0040-4039(95)02120-5
General Description
Odorless white to light tan solid. Sinks and mixes slowly with water.
Air & Water Reactions
Sublimes and forms vapor or dust that may explode [USCG, 1999].
Reactivity Profile
Salicylic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Salicylic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Hazard
Respiratory alkalosis, hyperkalemia, hyperthermia, dehydration, convulsions, shock, res- piratory paralysis, respiratory acidosis, lesions and death from respiratory collapse; fetotoxic.
Health Hazard
Inhalation of dust irritates nose and throat. Vomiting may occur spontaneously if large amounts are swallowed. Contact with eyes causes irritation, marked pain, and corneal injury which should heal. Prolonged or repeated skin contact may cause marked irritation or even a mild burn.
Biochem/physiol Actions
Salicylic acid (SA) plays a major role in the growth and development of plants. It also plays a role in endogenous signaling for plant disease resistance. SA is also involved in mediating thermogenesis and symptom development.
Mechanism of action
Salicylic acid has been shown to work through several different pathways. It produces its anti - inflammatory effects via suppressing the activity of cyclo oxygenase (COX), an enzyme which is responsible for the production of pro - inflammatory mediators such as the prostaglandins. Notably, it does this not by direct inhibition of COX, unlike most other non-steroidal anti-inflammatory drugs (NSAIDs), but instead by suppression of the expression of the enzyme (via a yet-un elucidated mechanism) . Salicylic acid has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK), and it is thought that this action may play a role in the anticancer effects of the compound and its prod rugs aspirin and salsalate. In addition, the anti diabetic effects of salicylic acid are likely mediated by AMPK activation primarily through allosteric conformational change that increases levels of phosphorylation. Salicylic acid also uncouples oxidative phosphorylation which leads to increased ADP:ATP and AMP:ATP ratios in the cell. Consequently, salicylic acid may alter AMPK activity and subsequently exert its anti-diabetic properties through altered energy status of the cell. Even in AMPK knock - out mice, however, there is an anti-diabetic effect demonstrating that there is at least one additional, yet - unidentified action of the compound.
Pharmacokinetics
Salicylic acid treats acne by causing skin cells to slough off more readily, preventing pores from clogging up. This effect on skin cells also makes salicylic acid an active ingredient in several shampoos meant to treat dandruff. Use of straight salicylic solution may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock. Subsalicylate in combination with bismuth form the popular stomach relief aid known commonly as Pepto-Bismol. When combined the two key ingredients help control diarrhea, nausea, heartburn, and even gas. It is also very mildly anti-biotic.
Pharmacology
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID). The main pharmacological effect is to inhibit prostaglandin metabolism and thromboxane synthesis by
inhibiting prostaglandin metabolism-required cyclooxygenase, via irreversible acetylation of 530 serine residues in the hydroxyl of COX-1 polypeptide chain,
which results in COX-1 inactivation, blocks the conversion of arachidonic acid into
thromboxane A2 pathway and then inhibits the platelet aggregation.
Prostaglandin is a hormone produced locally in the body. It can pass the pain to
the brain, regulate body temperature in the hypothalamus and cause inflammation.
Inhibition of prostaglandin synthesis can have antipyretic, analgesic, antiinflammatory and antirheumatic effects. The adverse effects of aspirin are mainly
gastrointestinal symptoms such as nausea, vomiting, upper abdominal discomfort or
pain. It can also cause allergic reactions, cardiotoxicity, liver and kidney damage
and Wright’s syndrome. In addition, high doses of aspirin can cause salicylic acid
reactions such as headache, dizziness, tinnitus, hearing loss and other central nervous system symptoms.
Clinical Use
The clinical application of aspirin varies with the therapeutic dose. Low-dose aspirin (75–300?mg/day) has antiplatelet aggregation effect and can be used to prevent and treat the coronary and cerebrovascular thrombosis and other postoperative thrombosis. The middle dose of aspirin (0.5–3? g/day) has antipyretic analgesic effects, so it is commonly used in the treatment of fever, headache, toothache, neuralgia, muscle pain and menstrual pain. High doses of aspirin (more than 4?g/day) have anti-inflammatory and antirheumatic effects for the treatment of acute rheumatic fever and rheumatoid arthritis. In addition, aspirin is used for the treatment of skin and mucous membrane lymphadenopathy (Kawasaki disease) in paediatric.
Side Effects
Salicylic acid's side effects include erythema and scaling.
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. Human systemic effects by skin contact: ear tinnitus. Mutation data reported. A skin and severe eye irritant. Experimental reproductive effects. Incompatible with iron salts, spirit nitrous ether, lead acetate, iodine. Used in the manufacture of aspirin. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Used as a topical keratolytic agent; in manufacture of aspirin, salicylates, resins, as a dyestuff intermediate; prevulcanization inhibitor; analytical reagent; fungicide, antiseptic, and food preservative.
Veterinary Drugs and Treatments
Often combined with sulfur, salicylic acid shampoos are often employed to treat patients with seborrheic disorders (seborrhea sicca and
oleosa) exhibiting mild to moderate scaling, with mild waxy and keratinous debris. In higher concentrations, topicals such as Kerasolv?
Gel (6.6% salicylic acid) can be used to remove localized excessive tissues associated with hyperkeratotic disorders, such as calluses and
idiopathic thickening of the planum nasale and footpads.
Salicylic acid has mildly antipruritic, antibacterial (bacteriostatic), keratoplastic and keratolytic actions. Lower concentrations are
primarily keratoplastic and higher concentrations, keratolytic. Salicylic acid lowers skin pH, increases corneocyte hydration and dissolves
the intercellular binder between corneocytes. Salicylic acid and sulfur are thought to be synergistic in their keratolytic actions.
Purification Methods
It has been purified by steam distillation, by recrystallisation from H2O (solubility is 0.22% at room temperature and 6.7% at 100o), absolute MeOH, or cyclohexane and by sublimation in a vacuum at 76o. The acid chloride (needles) has m 19-19.5o, b 92o/15mm, the amide has m 133o (yellow needles from H2O), the O-acetyl derivative has m 135o (rapid heating and the liquid resolidifies at 118o), and the O-benzoyl derivative has m 132o (aqueous EtOH). [IR: Hales et al. J Chem Soc 3145 1954, Bergmann et al. J Chem Soc 2351 1950]. [Beilstein 10 IV 125.]
Plant hormone
Salicylic acid (SA) is a phenolic phytohormone and is found in plants with roles in plant growth and development, photosynthesis, transpiration, ion uptake and transport. SA also induces specific changes in leaf anatomy and chloroplast structure. SA is involved in endogenous signaling, mediating in plant defense against pathogens. It plays a role in the resistance to pathogens by inducing the production of pathogenesis-related proteins . It is involved in the systemic acquired resistance (SAR) in which a pathogenic attack on one part of the plant induces resistance in other parts. The signal can also move to nearby plants by salicylic acid being converted to the volatile ester, methyl salicylate.
Incompatibilities
iron salts; lead acetate; iodine. Forms an explosive mixture in air.
Properties of Salicylic acid
| Melting point: | 158-161 °C(lit.) |
| Boiling point: | 211 °C(lit.) |
| Density | 1.44 |
| vapor density | 4.8 (vs air) |
| vapor pressure | 1 mm Hg ( 114 °C) |
| FEMA | 3985 | 2-HYDROXYBENZOIC ACID |
| refractive index | 1,565 |
| Flash point: | 157 °C |
| storage temp. | 2-8°C |
| solubility | ethanol: 1 M at 20 °C, clear, colorless |
| form | Solid |
| pka | 2.98(at 25℃) |
| color | White to off-white |
| PH | 3.21(1 mM solution);2.57(10 mM solution);2.02(100 mM solution); |
| PH Range | Non0 uorescence (2.5) to dark blue 0 uorescence (4.0) |
| Odor | at 100.00 %. faint phenolic nutty |
| Water Solubility | 1.8 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| λmax | 210nm, 234nm, 303nm |
| Merck | 14,8332 |
| JECFA Number | 958 |
| Sublimation | 70 ºC |
| BRN | 774890 |
| Stability: | Stable. Substances to be avoided include oxidizing agents, strong bases, iodine, fluorine. Combustible. Sensitive to light. |
| Major Application | Semiconductors, nanoparticles, photoresists, lubricating oils, UV absorbers, adhesive, leather, cleaner, hair dye, soaps, cosmetics, pain medication, analgesics, antibacterial agent, treatment of dandruff, hyperpigmented skin, tinea pedis, onychomycosis, osteoporosis, beriberi, fungicidal skin disease, autoimmune disease |
| CAS DataBase Reference | 69-72-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 2-hydroxy-(69-72-7) |
| EPA Substance Registry System | Salicylic acid (69-72-7) |
Safety information for Salicylic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Salicylic acid
| InChIKey | YGSDEFSMJLZEOE-UHFFFAOYSA-N |
Salicylic acid manufacturer
JSK Chemicals
Clickchem Research LLP
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Aspirin EP Impurity C 98%View Details
Aspirin EP Impurity C 98%View Details -
 Salicylic Acid (Technical) 98%View Details
Salicylic Acid (Technical) 98%View Details -
 SALICYLIC ACID 99%View Details
SALICYLIC ACID 99%View Details -
 Salicylic acid 98%View Details
Salicylic acid 98%View Details -
 Salicylic acid 98%View Details
Salicylic acid 98%View Details -
 Salicylic acid CAS 69-72-7View Details
Salicylic acid CAS 69-72-7View Details
69-72-7 -
 Salicylic Acid CASView Details
Salicylic Acid CASView Details -
 99% Lamivudine EP Impurity CView Details
99% Lamivudine EP Impurity CView Details
69-72-7
