69-72-7
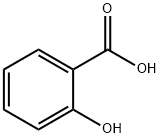
Product Name:
Salicylic acid
Formula:
C7H6O3
Synonyms:
2-Hydroxybenzoic acid;Acidum salicylicum;Salicylic Acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Salicylic acid is an odorless white to light tan solid. Sinks and mixes slowly with water. (USCG, 1999) |
|---|---|
| Color/Form | White crystals, fine needles, or fluffy white crystalline powder |
| Odor | SYNTHETIC ACID IS ODORLESS |
| Taste | Acrid taste |
| Boiling Point | 211 °C at 2.00E+01 mm Hg |
| Melting Point | 315 °F (USCG, 1999) |
| Flash Point | 315 °F (157 °C) (closed cup) |
| Solubility | 2240 mg/L (at 25 °C) |
| Density | 1.44 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 4.8 (Air= 1) |
| Vapor Pressure | 0.000082 [mmHg] |
| LogP | 2.26 |
| LogS | -1.82 |
| Stability/Shelf Life | GRADUALLY DISCOLORS IN SUNLIGHT |
| Autoignition Temperature | 1004 °F (540 °C) |
| Decomposition | When heated to decomp it emits acrid smoke and irritating fumes. |
| Corrosivity | Non-corrosive |
| Heat of Combustion | 3.026 millijoules/mole |
| pH | pH of saturated solution: 2.4 |
| Ionization Efficiency | Negative |
| Refractive Index | Index of refraction: 1.565 at 25 °C/D |
| Caco2 Permeability | -4.79 |
| Dissociation Constants | 2.78 |
| Collision Cross Section | 123.36 Ų [M-H]- [CCS Type: DT, Method: stepped-field] |
| Kovats Retention Index | 1308 1308 1330 1266 1277 1296 1305 1291 1263 1308 1330 1330 |
| Other Experimental Properties | Sublimes at 76 °C |
| Chemical Classes | Other Classes -> Benzoic Acid Derivatives |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 138.12 g/mol |
|---|---|
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 138.031694049 g/mol |
| Monoisotopic Mass | 138.031694049 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Salicylic acid is an odorless white to light tan solid. Sinks and mixes slowly with water. (USCG, 1999)
Uses
Salicylic acid is a medicated topical gel, cream, lotion or solution. It treats many skin disorders, such as acne, dandruff, psoriasis, seborrheic dermatitis of the skin and scalp, calluses, corns, common warts, and plantar warts, depending on the dosage form and strength of the preparation.
Adverse Effects
Although salicylic acid is considered safe overall, it may cause skin irritation when first starting. It may also remove too much oil, resulting in dryness and potential irritation.
