Salicylaldoxime
Synonym(s):2-Hydroxybenzaldehyde oxime
- CAS NO.:94-67-7
- Empirical Formula: C7H7NO2
- Molecular Weight: 137.14
- MDL number: MFCD00002120
- EINECS: 202-353-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
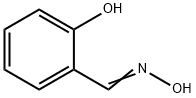
What is Salicylaldoxime?
Chemical properties
off-white to light beige crystalline powder
The Uses of Salicylaldoxime
Salicylaldoxime reacts with several metal ions to give intensively coloured
complexes which are insoluble in water. The structure of the copper(II), nickel(II)
and palladium(II) complexes is shown in Fig. 14 (structure I), and that of the
manganese(II), iron(II), cobalt(II) and zinc(II) complexes in Fig. 14 (structure
II). The copper(Il) and palladium(II) salicylaldoxime complexes are of importance
from an analytical point of view. The complexes precipitated from aqueous
media are easily filterable and washable and are weighed after drying at 100°C.
The precipitates are of stoichiometric composition.
The outstanding stabilities of the copper(II) and palladium(II) complexes
make the reagent suitable for the selective determination of these metal ions.
These two complexes form quantitatively at low pH values where the complexes
of other metal ions do not exist.
Besides the gravimetric method, salicylaldoxime is also useful in the volumetric,
amperometric and nephelometric determinations of copper and palladium.
Salicylaldoxime gives a black precipitate with VOs~ in media containing sulfuric
acid; this is soluble in chloroform to give an orange solution. The reaction is
specific for vanadium which can thus be detected by this method in the presence
of almost all other metals.
In neutral medium salicylaldoxime reacts with iron(III) to give a red complex
which is soluble in water. The reaction is utilized for the detection of micro
amounts of iron.
The Uses of Salicylaldoxime
As a reagent for copper and nickel.
Properties of Salicylaldoxime
| Melting point: | 57-59 °C
59-61 °C (lit.) |
| Boiling point: | 251.96°C (rough estimate) |
| Density | 1.2528 (rough estimate) |
| refractive index | 1.5030 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | sparingly soluble in water, soluble in ethanol, diethyl ether, acetone, benzene. |
| pka | pK1:1.37(+1);pK2:9.18;pK3:12.11 (25°C) |
| form | Powder |
| color | Bordeaux to purple-red |
| Odor | Odorless |
| Water Solubility | Slightly soluble in water |
| Merck | 14,8327 |
| BRN | 1859881 |
| CAS DataBase Reference | 94-67-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde, 2-hydroxy-, oxime(94-67-7) |
| EPA Substance Registry System | Benzaldehyde, 2-hydroxy-, oxime (94-67-7) |
Safety information for Salicylaldoxime
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Salicylaldoxime
| InChIKey | ORIHZIZPTZTNCU-VMPITWQZSA-N |
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran


You may like
-
 Salicylaldoxime 98%View Details
Salicylaldoxime 98%View Details -
 Salicylaldoxime 98%View Details
Salicylaldoxime 98%View Details -
 Salicylaldoxime 98% CAS 94-67-7View Details
Salicylaldoxime 98% CAS 94-67-7View Details
94-67-7 -
 94-67-7 Salicylaldoxime 98%View Details
94-67-7 Salicylaldoxime 98%View Details
94-67-7 -
 Salicylaldoxime, GR 98%+ CAS 94-67-7View Details
Salicylaldoxime, GR 98%+ CAS 94-67-7View Details
94-67-7 -
 Salicylaldoxime CAS 94-67-7View Details
Salicylaldoxime CAS 94-67-7View Details
94-67-7 -
 SALICYLADOXIME AR CAS 94-67-7View Details
SALICYLADOXIME AR CAS 94-67-7View Details
94-67-7 -
 Salicylaldoxime CAS 94-67-7View Details
Salicylaldoxime CAS 94-67-7View Details
94-67-7
