Ruthenium tetroxide
- CAS NO.:20427-56-9
- Empirical Formula: O4Ru
- Molecular Weight: 165.07
- MDL number: MFCD00074857
- EINECS: 243-813-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-28 13:53:21
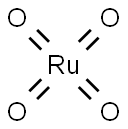
What is Ruthenium tetroxide?
Chemical properties
Ruthenium tetroxide is a golden-yellow volatile solid with an acrid odor, sparingly soluble in water and freely soluble in CCl4, in which it forms stable solutions. In comparison with the analogous compound OsO4, ruthenium tetroxide is a stronger oxidizing agent that reacts violently-resulting in explosion and/or flames-with most common organic solvents such as ether, alcohols, benzene, and pyridine, 1 and also with filter paper.

Ruthenium tetroxide is a clear orange to gold colored solution, It sublimes very easily at room temperature, possessing a melting point of 25.4℃ and a boiling point of 40℃ . While it is very soluble in CCl4 and other nonflammable organic solvents, a saturated solution in water at 20℃ reaches a concentration of 2% w/v.
The Uses of Ruthenium tetroxide
Ruthenium tetroxide is an oxidizing agent similar to osmium tetroxide, but more difficult to handle. The solvents normally employed in OsO4 oxidations (ether, benzene, pyridine) cannot be used because of their violent reaction with RuO4. Only CCl4 is recommended.
Preparation
Ruthenium tetroxide can be conveniently handled as a carbon tetrachloride solution that is easily prepared by stirring an aqueous solution of sodium periodate NaIO4 with a suspension of hydrated ruthenium dioxide in CCl4. Ruthenium tetroxide partitions between CCl4 and water, resulting in a 60:1 concentration ratio.
Production Methods
Ruthenium(VIII) oxide (ruthenium tetraoxide) RuO4 is formed when an alkaline ruthenium solution is treated with a strong oxidant, such as chlorine, or bromate ion when the Ru is in acid solution.
Hazard
Fire risk in contact with organic materials.
Toxicity evaluation
Ruthenium tetroxide(RuO4) is a toxic and explosive compound, and, although it is less toxic than OsO4, it must be handled in a well ventilated fume hood using goggles and gloves. It can be destroyed with a sodium bisulfite solution, resulting in the much safer and less toxic ruthenium dioxide, which is a dark insoluble solid with very low vapor pressure.
Properties of Ruthenium tetroxide
| Melting point: | 25.4° |
| Boiling point: | bp 40° |
| Density | 3.290 |
| storage temp. | Refrigerator (+4°C) |
| solubility | slightly soluble in H2O; very soluble in ctc; reacEtOH |
| form | Crystalline Powder |
| color | White |
| Water Solubility | 2.03g/100mL H2O (20°C); very soluble CCl4, other chlorinated hydrocarbons [MER06] [KIR82] |
| Sensitive | heat sensitive, store cold |
| Stability: | Stable. |
| CAS DataBase Reference | 20427-56-9 |
Safety information for Ruthenium tetroxide
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Ruthenium tetroxide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1