Platinum dioxide
- CAS NO.:1314-15-4
- Empirical Formula: O2Pt
- Molecular Weight: 227.08
- MDL number: MFCD00011184
- EINECS: 215-223-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-21 19:13:37
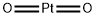
What is Platinum dioxide ?
Description
PTO2 is an alternating wide band-gap copolymer with electron-donating benzodithiophene (BDT) and electron-withdrawing thiophene carboxylate ester as the main backbone units. PTO2 enjoys good solubility in non-halogenated solvents, i.e. xylenes and THF.
Chemical properties
Platinum dioxide, also known as Adams catalyst, is a dark brown powder, a hydrated form of platinum (IV) oxide (PtO2), produced by heating chloroplatinic acid (H2PtCl6) with sodium nitrate (NaNO3). Platinum nitrate is produced, and this decomposes to Platinum (IV) oxide with evolution of NO2 and oxygen. It is used in hydrogenations of alkenes to alkanes, nitro compounds to aminos, and ketones to alcohols. The actual catalyst is not the oxide but finely divided platinum black, which forms during the hydrogenation reaction.
The Uses of Platinum dioxide
As catalyst in hydrogenations. The actual catalyst is platinum black which is formed in situ by reduction of the PtO2 by the hydrogen used for the hydrogenation. Especially useful for reduction at room tempereture and hydrogen pressures up to 4 atmospheres. Suitable for the reduction of double and triple bonds, aromatic nuclei, carbonyl groups, nitro groups, and nitriles.
The Uses of Platinum dioxide

A solution of the SM (2.435 g, 5.99 mmol) in EtOH (20 mL) was treated with PtO2 (0.136 g, 0.599 mmol), and was placed under 45 psi H2 for 1 h. LCMS indicated mostly product and de-brominated product. The mixture was diluted with DCM and filtered through celite. The filtrate was concentrated then purified directly by column chromatography (0-15% EtOAc/heptane) to provide the product. [0.970 g, 39.6%]
The Uses of Platinum dioxide
Platinum(IV) oxide is used as catalyst for hydrogenation reactions. It is applied in the hydrogenation of alkenes to alkanes, nitro compounds to amines and ketones to alcohols. It is also involved in the hydrogenolysis of cyclopropyl group in to an isopropyl group and in selective oxidation of primary alcohols.
Production Methods
PtO2 is obtained by reduction in chloroplatinic acid with formaldehyde or by fusing chloroplatinic acid with sodium nitrate.
What are the applications of Application
Platinum (IV) Oxide commonly referred to as Adam’s Catalyst is used frequently in organic synthesis reactions, mainly catalytic hydrogenation. The compound itself shows no cytotoxicity in cells of the pulmonary origin when compared to its PtCl4 counterpart. It has also been used as a catalyst in amperometric biosensors that are based on oxidase enzymes.
General Description
Platinum(IV) oxide has been used for the reduction of citernyl bromide to form (S)-3,7-dimethyloctylbromide. Prepared by Adams′ nitrate fusion method.
Properties of Platinum dioxide
| Melting point: | 450 °C (lit.) |
| Density | 10.2 |
| RTECS | TP2506020 |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | crystalline |
| appearance | Black solid |
| color | Dark brown |
| Water Solubility | Soluble in caustic potash solution. Insoluble in water, acid, aquaregia. |
| Merck | 14,7527 |
| Stability: | Contact with combustible material may cause fire. Incompatible with organic materials, powdered metals. |
| InChI | InChI=1S/2O.Pt |
| CAS DataBase Reference | 1314-15-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Platinum dioxide(1314-15-4) |
| EPA Substance Registry System | Platinum oxide (PtO2) (1314-15-4) |
| solubility | THF, o-xylene, chloroform, chlorobenzene and dichlorobenzene |
Safety information for Platinum dioxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Platinum dioxide
| InChIKey | WNVYCMIJBVIKSY-UHFFFAOYSA-N |
| SMILES | [Pt](=O)=O |
Platinum dioxide manufacturer
JSK Chemicals
Vineeth Precious Catalysts Pvt. Ltd.
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


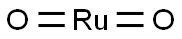

You may like
-
 Platinum Oxide 98%View Details
Platinum Oxide 98%View Details -
 Platinum dioxide 99%View Details
Platinum dioxide 99%View Details -
 Platinum(IV) oxide, Anhydrous CAS 1314-15-4View Details
Platinum(IV) oxide, Anhydrous CAS 1314-15-4View Details
1314-15-4 -
 Platinum(IV) oxide, Anhydrous CAS 1314-15-4View Details
Platinum(IV) oxide, Anhydrous CAS 1314-15-4View Details
1314-15-4 -
 Platinum(IV) oxide 81-83% Pt CAS 1314-15-4View Details
Platinum(IV) oxide 81-83% Pt CAS 1314-15-4View Details
1314-15-4 -
 Platinum(IV) oxide CAS 1314-15-4View Details
Platinum(IV) oxide CAS 1314-15-4View Details
1314-15-4 -
 Platinum(IV) oxide CAS 1314-15-4View Details
Platinum(IV) oxide CAS 1314-15-4View Details
1314-15-4 -
 Platinum(IV) Oxide CAS 1314-15-4View Details
Platinum(IV) Oxide CAS 1314-15-4View Details
1314-15-4
