Rubidium hydroxide
- CAS NO.:1310-82-3
- Empirical Formula: HORb
- Molecular Weight: 102.48
- MDL number: MFCD00011194
- EINECS: 215-186-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
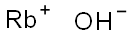
What is Rubidium hydroxide?
Chemical properties
clear colorless solution
Physical properties
Grayish-white orthogonal crystals; hygroscopic; density 3.2 g/cm3; melts at 301°C; very soluble in water (100 g/100 mL at 15°C), the solution highly alkaline; soluble in ethanol.
The Uses of Rubidium hydroxide
Rubidium Hydroxide is used to give fireworks a violet color. It is a notable ntermediate in the synthesis of other rubidium compounds. It is used in photography and storage batteries.
The Uses of Rubidium hydroxide
Rubidium hydroxide is an important intermediate in the synthesis of other rubidium compounds and can be used as a catalyst in oxidative chlorination reactions. It is also used in photography and storage batteries. Adding Rubidium hydroxide to fireworks to give it a violet color.
Preparation
Rubidium hydroxide may be obtained as an intermediate in recovering rubidium metal from mineral lepidolite (see Rubidium). In the laboratory it may be prepared by adding barium hydroxide to a solution of rubidium sulfate. The insoluble barium sulfate is separated by filtration:
Rb2SO4 + Ba(OH)2 → 2RbOH + BaSO4
Preparation should be in nickel or silver containers because rubidium hydroxide attacks glass. The solution is concentrated by partial evaporation. The commercial product is usually a 50% aqueous solution.
Definition
ChEBI: Rubidium hydroxide is an alkali metal hydroxide and a rubidium molecular entity.
General Description
A clear aqueous solution. Very caustic. Very irritating to skin and eyes. Used in electric storage batteries.
Air & Water Reactions
Absorbs carbon dioxide from the air. May generate large amounts of heat when diluted with water.
Reactivity Profile
A strong base. Forms caustic solution in water [Merck 11th ed. 1989].
Hazard
Strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Moderately toxic by ingestion. A powerful, corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of RbzO. See also POTASSIUM HYDROXIDE and RUBIDIUM.
Properties of Rubidium hydroxide
| Melting point: | 300-302°C |
| Density | 1.74 g/mL at 25 °C |
| solubility | soluble in ethanol |
| form | Liquid |
| color | Pale yellow |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,8285 |
| CAS DataBase Reference | 1310-82-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Rubidium monohydroxide(1310-82-3) |
| EPA Substance Registry System | Rubidium hydroxide (Rb(OH)) (1310-82-3) |
Safety information for Rubidium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Rubidium hydroxide
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


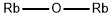
You may like
-
 Rubidium hydroxide solution CAS 1310-82-3View Details
Rubidium hydroxide solution CAS 1310-82-3View Details
1310-82-3 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1