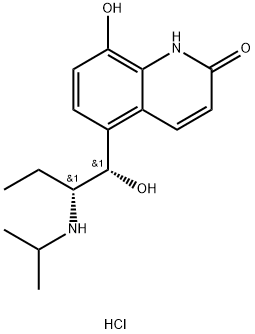
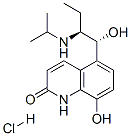
(R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone
- CAS NO.:72332-33-3
- Empirical Formula: C16H22N2O3
- Molecular Weight: 290.36
- MDL number: MFCD00867041
- EINECS: 276-590-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
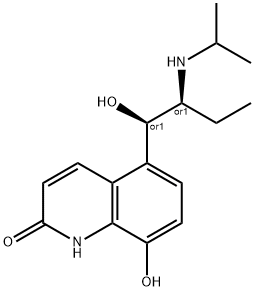
What is (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone?
Absorption
Because of the small therapeutic dose, systemic levels of salmeterol are low or undetectable after inhalation of recommended doses.
Toxicity
Symptoms of overdose include angina (chest pain), dizziness, dry mouth, fatigue, flu-like symptoms, headache, heart irregularities, high or low blood pressure, high blood sugar, insomnia, muscle cramps, nausea, nervousness, rapid heartbeat, seizures, and tremor.
Description
Like pirbuterol, procaterol exhibits similar broncholytic properties as albuteral, but it has somewhat of a more prolonged action. It is recommended for use as an inhaled drug for treating bronchial asthma.
Chemical properties
Off-White Solid
The Uses of (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone
A labelled intermediate of the enantiomer of Florfenicol (F405750), an antibacterial agent.
Background
A long-acting beta-2-adrenergic receptor agonist. It is a potent bronchodilator that may be administered orally or by aerosol inhalation.
Indications
For the treatment of asthma and chronic obstructive pulmonary disease (COPD).
Definition
ChEBI: 8-hydroxy-5-[1-hydroxy-2-(propan-2-ylamino)butyl]-1H-quinolin-2-one is a member of quinolines.
brand name
Pro-Air (Parke-Davis).
Pharmacokinetics
Procaterol is a long-acting beta-2-adrenergic receptor agonist. It is a potent bronchodilator that may be administered orally or by aerosol inhalation.
Synthesis
Procaterol, 5-[1-hydroxy-2-[(1-methylethyl)amino]butyl]-8-hydroxy-2-(1H) quinolone (23.3.25), is synthesized by acylation of 8-hydroxy-2(1H)-quinolone with 2-bromobutyric acid chloride at the fifth position of the quinoline system, which gives the compound 23.3.23. This undergoes action of isopropylamine, forming an aminoketone 23.3.24, the carbonyl group of which is reduced by sodium borohydride, giving procaterol (23.3.25) .
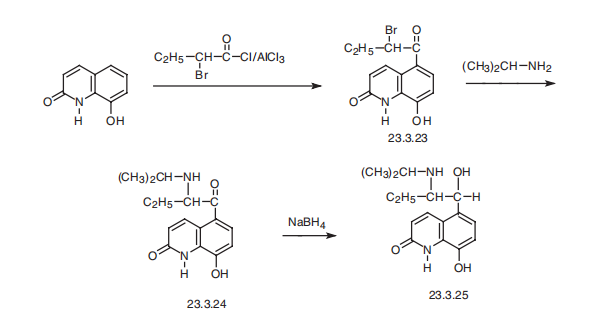
Metabolism
Not Available
Properties of (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone
| Melting point: | 107-109°C |
| storage temp. | -20°C |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), Methanol |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 72332-33-3(CAS DataBase Reference) |
Safety information for (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone
Computed Descriptors for (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone
(R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone manufacturer
New Products
tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-Arg-OH HCl H2O Indole Methyl Resin 2-CTC Resin Gabapentin EP Impurity A 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 1,3-Diphenylurea 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID N-METHYL INDAZOLE-3-CARBOXYLIC ACID 2-Hydroxy-5-nitroacetophenone 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2-HYDROXY BENZYL CYANIDE DIETHYL AMINOMALONATE HYDROCHLORIDE 5-BROMO-2CYANO PYRIDINE Boldenone Clarithromycin Ethinyl Estradiol StanozololRelated products of tetrahydrofuran


You may like
-
 ANTI-SARS VIRUS SN antibody produced in rabbit CASView Details
ANTI-SARS VIRUS SN antibody produced in rabbit CASView Details -
 CIS BROMO BENZOATE 98%View Details
CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 3-NITRO 2-METHYL BENZOIC ACID 98%View Details
3-NITRO 2-METHYL BENZOIC ACID 98%View Details
1975-50-4 -
 INDAZOLE-3-CARBOXYLIC ACID 4498-67-3 98%View Details
INDAZOLE-3-CARBOXYLIC ACID 4498-67-3 98%View Details
4498-67-3 -
 221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 -
 2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 -
 42831-50-5 98%View Details
42831-50-5 98%View Details
42831-50-5
