Rosiglitazone
Synonym(s):5-[[4-[2-(Methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione;Rosiglitazone
- CAS NO.:122320-73-4
- Empirical Formula: C18H19N3O3S
- Molecular Weight: 357.43
- MDL number: MFCD00871760
- EINECS: 924-121-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
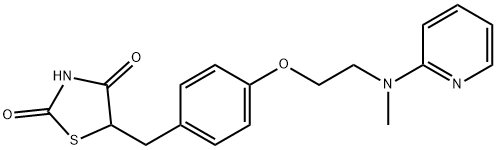
What is Rosiglitazone?
Absorption
The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Administration of rosiglitazone with food resulted in no change in overall exposure (AUC), but there was an approximately 28% decrease in Cmax and a delay in Tmax (1.75 hours). These changes are not likely to be clinically significant; therefore, rosiglitazone may be administered with or without food. Maximum plasma concentration (Cmax) and the area under the curve (AUC) of rosiglitazone increase in a dose-proportional manner over the therapeutic dose range.
Toxicity
Side effects include fluid retention, congestive heart failure (CHF), liver disease
Description
Rosiglitazone is an antidiabetic drug. The maleic acid salt of its racemic mixture is sold by GlaxoSmithKline under the trade name Avandia. In July,?the FDA voted to keep Avandia available?despite evidence linking it to an increased risk of heart disease. In September, FDA limited its use, and the?European Medicines Agency suspended marketing of the drug.
The Uses of Rosiglitazone
Rosiglitazone is an insulin sensitizer; binds to peroxisome proliferator activated receptor gamma (PPAR- γ).
The Uses of Rosiglitazone
antidiabetic
The Uses of Rosiglitazone
A potent and selective PPARγ agonist.
Indications
Rosiglitazone is approved for use as monotherapy and in conjunction with metformin, though it is sometimes combined with a sulfonylurea or insulin. It is usually taken once or twice a day with or without food. Rosiglitazone may cause a modest increase in lowdensity lipoprotein and triglyceride concentrations, but it is unclear whether this effect has any clinical significance or persists in the long term.
Indications
Rosiglitazone is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
What are the applications of Application
Rosiglitazone is a potent and selective PPARγ agonist that exhibits anti-diabetic and hepatoxic effects.
Background
Rosiglitazone is an anti-diabetic drug in the thiazolidinedione class of drugs. It is marketed by the pharmaceutical company GlaxoSmithKline as a stand-alone drug (Avandia) and in combination with metformin (Avandamet) or with glimepiride (Avandaryl). Like other thiazolidinediones, the mechanism of action of rosiglitazone is by activation of the intracellular receptor class of the peroxisome proliferator-activated receptors (PPARs), specifically PPARγ. Rosiglitazone is a selective ligand of PPARγ, and has no PPARα-binding action. Apart from its effect on insulin resistance, it appears to have an anti-inflammatory effect: nuclear factor kappa-B (NFκB) levels fall and inhibitor (IκB) levels increase in patients on rosiglitazone. Recent research has suggested that rosiglitazone may also be of benefit to a subset of patients with Alzheimer's disease not expressing the ApoE4 allele. This is the subject of a clinical trial currently underway.
Definition
ChEBI: Rosiglitazone is an aminopyridine and a member of thiazolidinediones. It has a role as an insulin-sensitizing drug, a ferroptosis inhibitor and an EC 6.2.1.3 (long-chain-fatty-acid--CoA ligase) inhibitor. It is a conjugate acid of a rosiglitazone(1-).
brand name
Avandia (GlaxoSmithKline).
General Description
Rosiglitazone is 5-[4-[2-(N-methyl-N-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione, and is availableas the maleate salt in tablets containing the drug alone(Avandia) or in combination products with metformin(Avandamet) or with glimepiride (Avandaryl). The2-aminopyridine moiety allows for salt formation; the marketedformulations contain the 1:1 salt with maleic acid, inwhich the pyridine nitrogen accepts a proton, forming the2-aminopyridinium species.
General Description
Rosiglitazone, (±)-5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione(Avandia), is a white to off-white solid with pKavalues of 6.8 and 6.1. Rosiglitazone is readily soluble inethanol and a buffered aqueous solution with pH of 2.3; solubilitydecreases with increasing pH in the physiologicalrange. The molecule has a single chiral center and is presentas a racemate. Even so, the enantiomers are functionally indistinguishablebecause of rapid interconversion.
Biological Activity
rosiglitazone is a potent agonist of peroxisome proliferator-activated receptor γ (pparγ), a subfamily of the nuclear-receptor superfamily which is predominately expressed in adipose tissue and regulates gene expression responding to ligand binding. belonging to the thiazolidinedione (tzd) class, rosiglitazone, like other tzd members, binds to pparγ dna as heterodimers and activate transcription of various metabolic regulators involved in the differentiation of stem cells into adipocytes and increased expression of genes regulating the metabolism of glucose and lipid. rosiglitazone is used to treat patients with type ii diabetes mellitus for its strong ability to improve insulin sensitization through its effects either on fatty acid uptake and storage in adipose tissue or on adiokines.peter j. cox, david a. ryan, frank j. hollis, ann-marie harris, ann k. miller, marika vousden and hugh cowley. absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. drug metabolism and disposition 2000; 28 (7): 772-780adie vilioen and alan sinclair. safety and efficacy of rosiglitazone in the elderly diabetic patient. vascular health and risk management 2009: 5 389-395
Biochem/physiol Actions
Rosiglitazone is a potent agonist for PPARγ with an EC50 of 43 nM for the human receptor. It is antidiabetic, working as an insulin sensitizer by binding to the PPARγ receptors in fat cells and making the cells more responsive to insulin.
Pharmacokinetics
When rosiglitazone is used as monotherapy, it is associated with increases in total cholesterol, LDL, and HDL. It is also associated with decreases in free fatty acids. Increases in LDL occurred primarily during the first 1 to 2 months of therapy with AVANDIA and LDL levels remained elevated above baseline throughout the trials. In contrast, HDL continued to rise over time. As a result, the LDL/HDL ratio peaked after 2 months of therapy and then appeared to decrease over time.
Clinical Use
Although rosiglitazone is extensively biotransformed—playing the major role in both transformations, and some involvementof CYP2C9.21,47 The sulfate conjugate M10 is thepredominant circulating metabolite by 4-hour postdose. Theextraordinarily high plasma protein binding of this metabolite(and the N-demethylated sulfate conjugate M4) in humans accountsfor the lengthy residence time of the radioactivity in thebody, despite the relatively short pharmacokinetic half-life(4–4.5 hours) of rosiglitazone itself.
Metabolism
Hepatic. Rosiglitazone is extensively metabolized in the liver to inactive metabolites via N-demethylation, hydroxylation, and conjugation with sulfate and glucuronic acid. In vitro data have shown that Cytochrome (CYP) P450 isoenzyme 2C8 (CYP2C8) and to a minor extent CYP2C9 are involved in the hepatic metabolism of rosiglitazone.
Storage
+4°C
Properties of Rosiglitazone
| Melting point: | 153-155 C |
| Boiling point: | 585.0±35.0 °C(Predicted) |
| Density | 1.315±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥10mg/mL |
| form | powder |
| pka | 6.34±0.50(Predicted) |
| color | White to Off-White |
| Merck | 14,8265 |
| CAS DataBase Reference | 122320-73-4(CAS DataBase Reference) |
| IARC | 3 (Vol. 108) 2016 |
Safety information for Rosiglitazone
Computed Descriptors for Rosiglitazone
Rosiglitazone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Rosiglitazone CAS 122320-73-4View Details
Rosiglitazone CAS 122320-73-4View Details
122320-73-4 -
 Rosiglitazone 98% CAS 122320-73-4View Details
Rosiglitazone 98% CAS 122320-73-4View Details
122320-73-4 -
 Rosiglitazone CAS 122320-73-4View Details
Rosiglitazone CAS 122320-73-4View Details
122320-73-4 -
 Rosiglitazone CAS 122320-73-4View Details
Rosiglitazone CAS 122320-73-4View Details
122320-73-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
