Pioglitazone hydrochloride
Synonym(s):5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione monohydrochloride;5-[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]benzyl]thiazolidine-2,4-dione hydrochloride;Pioglitazone hydrochloride
- CAS NO.:112529-15-4
- Empirical Formula: C19H21ClN2O3S
- Molecular Weight: 392.9
- MDL number: MFCD04975446
- EINECS: 629-731-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
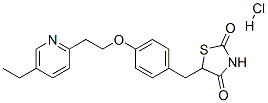
What is Pioglitazone hydrochloride?
Description
Pioglitazone is a new orally active thiazolidinedione (TZD) launched in the US for the treatment of non-insulin dependent diabetes mellitus (NIDDM). It can be synthesized in 4 steps, the last one transforming an alphabromoester into thiazolidine with thiourea. As with other representatives in this class, it potently activates the nuclear receptor peroxisome proliferator-activated receptor gamma which is believed to be involved in the regulation of insulin resistance and adipogenesis. In several obese and obese diabetic animal models, treatment with Pioglitazone resulted in reductions in plasma glucose and serum lipids. In clinical studies, Pioglitazone at a once daily oral dose of 15-45 mg, as monotherapy or in combination with non-TZDs or insulin, was shown to significantly improve glycemic control in type-2 diabetes and demonstrated a beneficial effect on insulin resistance and other clinically relevant parameters as plasma levels of triglycerides or HDL-cholesterol. Pioglitazone is reported to be safe and well tolerated and is said to have a lower occurrence of hepatic toxicity as well as a low probability for drug interaction.
Chemical properties
Colourless Prisms
Originator
Takeda (Japan)
The Uses of Pioglitazone hydrochloride
Pioglitazone hydrochloride is a euglycemic agent,used as an antidiabetic.
The Uses of Pioglitazone hydrochloride
Pioglitazone Hydrochloride is used as an antidiabetic.
Definition
ChEBI: Pioglitazone hydrochloride is an aromatic ether.
Manufacturing Process
To a solution of 2-(5-ethyl-2-pyridyl)ethanol (53.0 g) and 4-fluoronitrobenzene (47.0 g) in DMF (500 ml) was added portionwise under ice-cooling 60% sodium hydride in oil (16.0 g). The mixture was stirred under ice-cooling for one hour, then at room temperature for 30 min, poured into water and extracted with ether. The ether layer was washed with water and dried (MgSO4). The solvent was evaporated off to give 4-[2-(5-ethyl-2pyridyl)ethoxy]nitrobenzene as crystals (62.0 g, 62.9%). Recrystallization from ether-hexane gave colorless prisms, melting point 53°-54°C.
A solution of 4-[2-(5-ethyl-2-pyridyl)ethoxy]nitrobenzene (60.0 g) in methanol (500 ml) was hydrogenated at room temperature under one atmospheric pressure in the presence of 10% Pd-C (50% wet, 6.0 g). The catalyst was removed by filtration and the filtrate was concentrated under reduced pressure. The residual oil was dissolved in acetone (500 ml)methanol (200 ml). To the solution was added a 47% HBr aqueous solution (152 g). The mixture was cooled, to which was added dropwise a solution of NaNO2 (17.3 g) in water (30 ml) at a temperature not higher than 5°C. The whole mixture was stirred at 5°C for 20 min, then methyl acrylate (112 g) was added thereto and the temperature was raised to 38°C. Cuprous oxide (2.0 g) was added to the mixture in small portions with vigorous stirring. The reaction mixture was stirred until nitrogen gas evolution ceased, and was concentrated under reduced pressure. The concentrate was made alkaline with concentrated aqueous ammonia, and extracted with ethyl acetate. The ethyl acetate layer was washed with water and dried (MgSO4) The solvent was evaporated off to leave methyl 2-bromo-3-{4-[2-(5-ethyl-2pyridyl)ethoxy]phenyl}propionate as a crude oil (74.09 g, 85.7%).
A mixture of the crude oil of methyl 2-bromo-3-{4-[2-(5-ethyl-2pyridyl)ethoxy]phenyl}propionate (73.0 g) thiourea (14.2 g), sodium acetate (15.3 g) and ethanol (500 ml) was stirred for 3 hours under reflux. The reaction mixture was concentrated under reduced pressure, and the concentrate was neutralized with a saturated aqueous solution of sodium hydrogencarbonate, to which were added water (200 ml) and ether (100 ml). The whole mixture was stirred for 10 min to yield 5-{4-[2-(5-ethyl-2pyridyl)ethoxy]benzyl}-2-imino-4-thiazolidinone as crystals (0.3 g, 523.0%). Recrystallization from methanol gave colorless prisms, melting point 187°188°C, dec.
A solution of 5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2-imino-4thiazolidinone (23.5 g) in 2 N HCl (200 ml) was refluxed for 6 hours. The solvent was evaporated off under reduced pressure, and the residue was neutralized with a saturated aqueous solution of sodium hydrogencarbonate. The crystals (23.5 g, 97.5%) which precipitated were collected by filtrationand recrystallized from DMF-H2O to give 5-{4-[2-(5-ethyl-2pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione as colorless needles (20.5 g, 86.9%), melting point 183°-184°C.
In practice it is usually used as hydrochloride salt.
Mechanism of action
Pioglitazone hydrochloride works by helping to restore your body's proper response to insulin, thereby lowering your blood sugar.Controlling high blood sugar helps prevent kidney damage, blindness, nerve problems, loss of limbs, and sexual function problems. Proper control of diabetes may also lessen your risk of a heart attack or stroke.
brand name
Actos (Takeda).
Therapeutic Function
Antidiabetic
Side Effects
Pioglitazone hydrochloride may cause serious side effects including:
shortness of breath (especially when laying down),
unusual tiredness,
swelling,
rapid weight gain,
pink or red urine,
painful or difficult urination,
new or worsening urge to urinate,
vision changes, and
sudden, unusual pain in your hand, arm or foot.
General Description
Pioglitazone hydrochloride is an oral antidiabetic agent used in the treatment of type 2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus (NIDDM) or adult-onset diabetes.
Biochem/physiol Actions
Selective PPARγ agonist
storage
room temperature (desiccate)
References
1) Merck 14:7452 2) Sakamoto et al. (2000), Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone; Biochem. Biophys. Res. Commun., 278 704 3) Wilson et al. (2000), The PPARs: From Orphan Receptors to Drug Discovery; J. Med. Chem., 43 527 4) Shannon et al. (2017), Pioglitazone Inhibits Mitochondrial Pyruvate Metabolism and Glucose Production in Hepatocytes; FEBS J., 284 451 5) Zhao et al. (2016), The Antidepressant-Like Effects of Pioglitazone in a Chronic Mild Stress Mouse Model Are Associated With PPARγ-Mediated Alteration of Microglial Activation Phenotypes; Neuroinflamm., 13 259
Properties of Pioglitazone hydrochloride
| Melting point: | 193-194°C |
| RTECS | XJ5813440 |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥10mg/mL |
| form | powder |
| color | white to off-white |
| Water Solubility | Soluble in water (<1 mg/ml at 25°C), DMF, methanol, ethanol (4 mg/ml at 25°C), and DMSO (79 mg/ml at 25°C). |
| Merck | 14,7452 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 112529-15-4(CAS DataBase Reference) |
Safety information for Pioglitazone hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Pioglitazone hydrochloride
| InChIKey | GHUUBYQTCDQWRA-UHFFFAOYSA-N |
Pioglitazone hydrochloride manufacturer
AKASH PHARMA EXPORTS
SETV ASRV LLP
Global Chemie
Bazayan & Co.
New Products
3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 5-Amino Salicylicacid, 95% 4-(Cyanomethyl)benzoic acid, 95% High Vac grease Trimethylsilyl cyanide, 90% Phenyl dichlorophosphate,98% Decanoic acid,98% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH 2-Chloromethyl-4-methyl-quinazoline 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic Acid Trans-4-Aminocyclohexanol [4tac] 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] L-Glutamic Acid Diethyl Ester Hydrochloride Bis(2-Chloroethyl) Amine HydrochlorideRelated products of tetrahydrofuran
![4-Chloro-N-[2-[[5-(trifluoromethyl)-2-pyridinyl]sulfonyl]ethyl]benzamide](https://img.chemicalbook.in/CAS/GIF/188591-46-0.gif)

You may like
-
 Pioglitazone Hydrochloride 98%View Details
Pioglitazone Hydrochloride 98%View Details
112529-15-4 -
 Pioglitazone hydrochloride 99%View Details
Pioglitazone hydrochloride 99%View Details -
 Pioglitazone HCl 95.00% CAS 112529-15-4View Details
Pioglitazone HCl 95.00% CAS 112529-15-4View Details
112529-15-4 -
 Pioglitazone Hcl 99%View Details
Pioglitazone Hcl 99%View Details -
 PIOGLITAZONE HCL 112529-15-4 95-99%View Details
PIOGLITAZONE HCL 112529-15-4 95-99%View Details
112529-15-4 -
 Pioglitazone hydrochloride 98%View Details
Pioglitazone hydrochloride 98%View Details -
 Pioglitazone HCl >98% (HPLC) CAS 112529-15-4View Details
Pioglitazone HCl >98% (HPLC) CAS 112529-15-4View Details
112529-15-4 -
 Pioglitazone HCl 98% (HPLC) CAS 112529-15-4View Details
Pioglitazone HCl 98% (HPLC) CAS 112529-15-4View Details
112529-15-4
