(R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid
- CAS NO.:1072833-77-2
- Empirical Formula: C14H19BCl2N2O4
- Molecular Weight: 361.03
- MDL number: MFCD18251438
- EINECS: 810-246-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
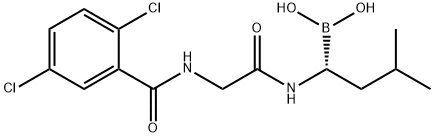
What is (R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid?
Absorption
After oral administration, the time to reach maximum concentration in plasma was 1 hour. The mean absolute oral bioavailability is 58%.
Toxicity
Drug-induced liver injury, hepatocellular injury, hepatic steatosis, hepatitis cholestatic and hepatotoxicity have each been reported in <1% of patients. Ixazomib can cause fetal harm when administered to pregnant women, and therefore it should also be advised to women of reproductive age to avoid becoming pregnant on ixazomib.
The Uses of (R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid
Ixazomib is a proteasome inhibitor that acts by preventing cell growth in solid tumours. It is an anticancer agent that is used to treat patients with multiple myeloma and is potentially neurotoxic.
Background
Ixazomib a second generation proteasome inhibitor (PI) and the first oral PI approved by the FDA in November 2015 for multiple myeloma treatment in combination with 2 other therapies (lenalidomide and dexamethasone) for patients who have received at least 1 prior therapy. It was found to have similar efficacy to bortezomib (the first PI approved for multiple myeloma therapy) in the control of myeloma growth and prevention of bone loss. Ixazomib citrate is marketed by Takeda Pharmaceuticals under the brand name Ninlaro, which is a prodrug that becomes quickly converted to its active metabolite, ixazomib, after administration.
Indications
Ixazomib is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
Definition
ChEBI: A glycine derivative that is the amide obtained by formal condensation of the carboxy group of N-(2,5-dichlorobenzoyl)glycine with the amino group of [(1R)-1-amino-3-methylbutyl]boronic acid. The active metabolite of ixa omib citrate, it is used in combination therapy for treatment of multiple myeloma.
Pharmacokinetics
In vitro studies have shown ixazomib to induce apoptosis in multiple myeloma cells sensitive or resistant to other conventional therapies. In mouse xenograft models, ixazomib induced tumor growth inhibition.
Metabolism
Metabolism of ixazomib is expected to be by CYP and non-CYP pathways, with no predominant CYP isozyme contribution. At higher than clinical concentrations, ixazomib was metabolized by multiple CYP isoforms with estimated relative contributions of 3A4 (42%), 1A2 (26%), 2B6 (16%), 2C8 (6%), 2D6 (5%), 2C19 (5%) and 2C9 (<1%).
Properties of (R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid
| Melting point: | >142°C (subl.) |
| Density | 1.306 |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Sparingly), Methanol (Slightly) |
| form | Solid |
| pka | 9.67±0.43(Predicted) |
| color | White to Pale Yellow |
Safety information for (R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid
Computed Descriptors for (R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Ixazomib 97%View Details
Ixazomib 97%View Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
