MLN9708
- CAS NO.:1201902-80-8
- Empirical Formula: C20H23BCl2N2O9
- Molecular Weight: 517.12
- MDL number: MFCD18251437
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33

What is MLN9708?
Description
Ixazomib citrate is a proteasome inhibitor prodrug for the treatment of multiple myeloma in patients who have received at least one prior therapy in combination with lenalidomide and dexamethasone. The drug was developed by Takeda and reversibly inhibits the protein proteasome subunit β type-5, which is part of the 20S proteasome complex. Ixazomib citrate (XXIV) is hydrolyzed quickly in vivo to give the biologically active compound ixazomib, which presumably is the corresponding boronic acid variant of XXIV.
The Uses of MLN9708
MLN-9708 is a novel proteasome?inhibitor.
Clinical Use
Highly selective and reversible proteasome inhibitor:
Treatment of multiple myeloma in combination with
lenalidomide and dexamethasone
Synthesis
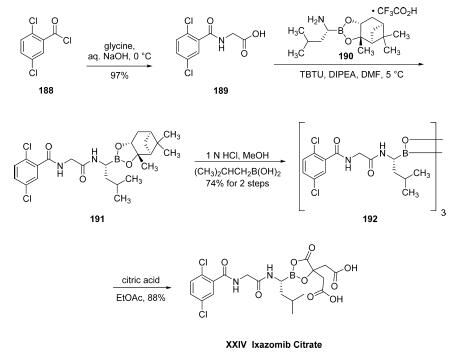
The structure of ixazomib citrate is particularly interesting in that it is one of the relatively few marketed drugs which feature a boron atom within its structure (others of note being the oncology medication bortezomib and the antifungal drug tavaborole13). The ostensible scale synthetic approach began with reaction of commercial 2,5-dichlorobenzoyl chloride (188) with glycine in aqueous NaOH to furnish amide 189 in 97% yield as a white crystalline solid. Acid 189 was then coupled with commercially available 1,3,2-benzodioxaborolane 190 in the presence of TBTU and DIPEA in DMF at low temperature to give diamide 191, which was used without purification for the next step. Borane 191 was then deprotected with (2-methylpropyl)boronic acid in methanolic HCl to provide trimer 192 in 74% as a white solid. Finally, boroxin 192 was reacted with citric acid in EtOAc to dissociate the trimer, resulting in ixazomib citrate (XXIV) in 88% yield as a crystalline solid.
Properties of MLN9708
| Melting point: | >227°C (dec.) |
| Density | 1.47 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 1.92±0.20(Predicted) |
| color | White to Off-White |
Safety information for MLN9708
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MLN9708
MLN9708 manufacturer
AKASH PHARMA EXPORTS
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
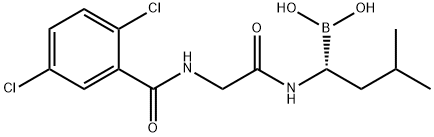
 Ixazomib Citrate 99%View Details
Ixazomib Citrate 99%View Details -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1