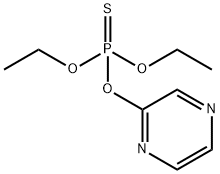
Quinalphos
- CAS NO.:13593-03-8
- Empirical Formula: C12H15N2O3PS
- Molecular Weight: 298.3
- MDL number: MFCD00078719
- EINECS: 237-031-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
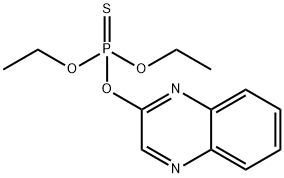
What is Quinalphos?
The Uses of Quinalphos
Quinalphos is used to control both chewing and sucking insects and mites in a large number of different crops.
Definition
ChEBI: Quinalphos is an organic thiophosphate and an organothiophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide and an agrochemical. It is functionally related to a quinoxalin-2-ol.
Safety Profile
Poison by ingestion, inhalation, skin contact, parenteral, and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. An insecticide. When heated to decomposition it emits very toxic fumes of NOx, POx, and SOx
Metabolic pathway
In methanol solution, quinalphos undergoes photodegradation reactions of isomerization, oxidation, and deesterification, together with other degradation reactions, resulting in several degradation products. However, the photolysis of quinalphos in ethanol solution yields the two products, O,O-diethyl-O-(3- ethoxyquinoxalin-2-yl)phosphorothioate and O,O- diethyl-O-[3(1-hydroxyethyl)-quinoxalin- 2yl]phosphorothioate, both of which are derived from the reaction of the photoexcited quinalphos molecule with the solvent.
Degradation
Quinalphos is susceptible to hydrolysis. The DT50 values at pH 3,6 and 9
were 23,39 and 26 days, respectively (PM). Quinalphos is very susceptible
to acid hydrolysis but stable under mildly basic conditions, having a halflife
ten times that of parathion at pH 11 (Schmidt, 1972). Quinalphos was
degraded in distilled water (pH 6.0), rain water (pH 6.8) and tap water
(pH 7.8) with DT50 values of 80,50 and 60 days, respectively (Dureja ef aI.,
1988). The photolysis of quinalphos in ethanolic solution irradiated by a
medium pressure mercury vapour lamp (λmax 366 nm) with a glass filter to
screen out light of <290 nm gave photoproducts principally derived from
reaction with the solvent. The main photolysis products were 3-ethoxyand
3-hydroxyethyl-quinoxaline derivatives of quinalphos (not shown in
Scheme 1). A minor product of photodegradation was quinalphos oxon
(2) (Pusino et al., 1989). When quinalphos was irradiated with a high
pressure mercury vapour lamp (λmax 254 nm) in a quartz reactor many
more photoproducts were detected, although whether this information is
relevant to photodegradation in the environment is debatable since light
of wavelength ﹤290 nm is generally absent at the Earth's surface. Using
this method of irradiation, Dureja et al. (1988) examined the photolysis of
quinalphos in hexane and methanol solution, as a thin film on a glass
plate and on the soil surface. Analysis of the photoproducts was by MS
and 1H NMR spectroscopy after separation by TLC and GC. The rates of
photodecomposition in aqueous solution and on soil surfaces exposed to
sunlight were also measured, although in these experiments the nature of
the photoproducts were not determined. In methanol solution, three
major pathways of photolysis were apparent: (a) de-esterification to
afford quinoxolin-2-o1(3), (b) thionethiolo rearangement to O,O-diethyl
S-quinoxalin-2-yl phosphorothioate (4), which was not actually detected
and was presumably de-esterified to give quinoxoline-2-thiol (5) which
dimerised to the disulfide (6) and the sulfide (7), and (c) oxidation of
the P=S sulfur to afford the oxon (2) as shown in Scheme 1. In addition,
the de-esterification of the phosphorothioate moiety and additional alkyl
transfer from quinalphos gave triethyl phosphate (S), O,O,O-triethyl
phosphorothioate (9) and O,O,S-triethyl phosphorodithioate (10). A
number of other photoproducts were identified which were formed via reaction of the methanol solvent with quinalphos (1) and quinoxolin-2-o1
(3) but because of their non-relevance to photoproducts produced under
environmental conditions they are not included in Scheme 1.
Irradiation of quinalphos as a thin film on glass produced quinalphos
oxon (2) and quinoxolin-2-o1(3) as the main photoproducts. The products
from the soil irradiation experiments were identified as quinalphos oxon
(2), the rearranged product O,O-diethyl S-quinoxalin-2-yl phosphorothioate
(4), quinoxolin-2-o1(3), quinoxoline-2-thiol(5) and the sulfide (7).
Routes for photodegradation of quinalphos in methanol solution, as a thin
film and on soil surfaces are shown in Scheme 1. Half-lives for the photodecomposition
of quinalphos by sunlight in water varied from 23 to 30
days and on soil surfaces from 2 to 5 days.
Properties of Quinalphos
| Melting point: | 31.5°C |
| Boiling point: | 142 °C |
| Density | 1.306±0.06 g/cm3(Predicted) |
| vapor pressure | 3.46×10-4
Pa (20 °C) |
| Flash point: | 2 °C |
| storage temp. | APPROX 4°C
|
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | -1.05±0.30(Predicted) |
| form | Crystalline |
| Water Solubility | 17.8 mg l-1 (22-23 °C) |
| BRN | 754823 |
| Stability: | Hygroscopic, Moisture sensitive |
| CAS DataBase Reference | 13593-03-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Quinalphos(13593-03-8) |
| EPA Substance Registry System | Quinalphos (13593-03-8) |
Safety information for Quinalphos
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H311:Acute toxicity,dermal H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Quinalphos
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 13593-03-8 99%View Details
13593-03-8 99%View Details
13593-03-8 -
 Quinalphos 98%View Details
Quinalphos 98%View Details
13593-03-8 -
 Quinalphos 98%View Details
Quinalphos 98%View Details
13593-03-8 -
 Quinalphos CAS 13593-03-8View Details
Quinalphos CAS 13593-03-8View Details
13593-03-8 -
 Quinalphos CAS 13593-03-8View Details
Quinalphos CAS 13593-03-8View Details
13593-03-8 -
 Quinalphos 25 E.C.View Details
Quinalphos 25 E.C.View Details
13593-03-8 -
 Powder Quinalphos 25% Arkayl Insecticide, 1 kgView Details
Powder Quinalphos 25% Arkayl Insecticide, 1 kgView Details
13593-03-8 -
 Liquid Quinalphos 25 Ec Insecticide, 250 mlView Details
Liquid Quinalphos 25 Ec Insecticide, 250 mlView Details
13593-03-8
