Ethylene glycol diethyl ether
- CAS NO.:629-14-1
- Empirical Formula: C6H14O2
- Molecular Weight: 118.17
- MDL number: MFCD00009253
- EINECS: 211-076-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:29
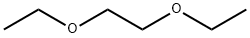
What is Ethylene glycol diethyl ether?
Description
Ethylene glycol diethyl ether is a colorless liquid. Molecular weight = 118.20; Boiling point = 122℃;Freezing/Melting point = - 74℃; Flash point= 35℃;Autoignition temperature = 406℃. Hazard Identification(based on NFPA-704 M Rating System): Health 1,Flammability 3, Reactivity 0. Slightly soluble in water.
Chemical properties
Clear colorless liquid
Chemical properties
Ethylene glycol diethyl ether is a colorless liquid.
The Uses of Ethylene glycol diethyl ether
Ethylene glycol diethyl ether is a important solvent, used for ink, paint and coating industry. Mainly used in polymer, electrochemistry, boracium chemistry, resin, nitro cellulose, In this product , the benzene, xenene, terphenyl, anthracene reacts with sodium and then bring about the complex; it is also used in surface treatment, halogen analysis in gasoline, acetic acid recycle in diluted acetic acid, depaint agent, thinner, flushing agent, stabilizer, anti oxidant , thickener of lube.
General Description
A clear colorless liquid with a faint ether-like odor. Flash point 95°F. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Ethylene glycol diethyl ether can react with oxidizers. Ethylene glycol diethyl ether is incompatible with strong acids.
Health Hazard
Inhalation causes irritation of nose and throat. Contact with liquid irritates eyes but has little or no effect on skin. Ingestion causes irritation of mouth and stomach.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by ingestion. Mildly toxic by inhalation. An experimental teratogen. Experimental reproductive effects. An eye irritant. An aprotic solvent. A very dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. To fight fire, use CO2, dry chemical. See also GLYCOL ETHERS and various cellosolve entries.
Potential Exposure
Ethylene glycol diethyl ether is used as an aprotic solvent; in chemical manufacturing; as a solvent for detergents and in other cleaning products
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.May form peroxides in storage. Prior to working with thischemical you should be trained on its proper handling andstorage. Ethylene glycol diethyl ether must be stored toavoid contact with strong oxidizers (such as chlorine, bromine, and fluorine) and strong acids (such as hydrochloric,sulfuric, and nitric) since violent reactions occur. Sources ofignition, such as smoking and open flames, are prohibitedwhere ethylene glycol diethyl ether is handled, used, orstored. Metal containers involving the transfer of 5 gallonsor more of ethylene glycol diethyl ether should be groundedand bonded. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters.Use only nonsparking tools and equipment, especially whenopening and closing containers of ethylene glycol diethylether. Wherever ethylene glycol diethyl ether is used, handled, manufactured, or stored, use explosion-proof electricalequipment and fittings.
Shipping
UN1153 Ethylene glycol diethyl ether, Hazard Class: 3; Labels: 3-Flammable liquid
Purification Methods
After refluxing for 12hours, a mixture of the ether (2L), conc HCl (27mL) and water (200mL), is added with slow passage of nitrogen. The solution is cooled, and KOH pellets are added slowly and with shaking until no more dissolves. The organic layer is decanted, treated with some KOH pellets and again decanted. It is then refluxed with, and distilled from sodium immediately before use. Alternatively, after removal of peroxides by treatment with activated alumina, the ether is refluxed in the presence of the blue ketyl formed by sodium-potassium alloy with benzophenone, then distilled. [Beilstein 1 H 468, 1 II 519, 1 III 2078, 1 IV 2379.]
Incompatibilities
Forms explosive mixture with air when heated. Incompatible with oxidizers (chlorates, nitrates,peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Attacks some plastics, rubber and coatings. May slowly form unstable reactive peroxides during prolonged storage or on exposure to air and light. Also incompatible with strong acids; aluminum and its alloys
Properties of Ethylene glycol diethyl ether
| Melting point: | -74 °C |
| Boiling point: | 121 °C(lit.) |
| Density | 0.842 g/mL at 25 °C(lit.) |
| vapor pressure | 9.4 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 69 °F |
| storage temp. | Store below +30°C. |
| solubility | 34g/l |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | Slightly soluble |
| BRN | 1732917 |
| CAS DataBase Reference | 629-14-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, 1,2-diethoxy-(629-14-1) |
| EPA Substance Registry System | Ethylene glycol diethyl ether (629-14-1) |
Safety information for Ethylene glycol diethyl ether
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Ethylene glycol diethyl ether
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 1,2-Diethoxyethane CAS 629-14-1View Details
1,2-Diethoxyethane CAS 629-14-1View Details
629-14-1 -
 1,2-Diethoxyethane CAS 629-14-1View Details
1,2-Diethoxyethane CAS 629-14-1View Details
629-14-1 -
 Ethylene glycol diethyl ether CAS 629-14-1View Details
Ethylene glycol diethyl ether CAS 629-14-1View Details
629-14-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1