PYRILAMINE MALEATE
Synonym(s):Mepyramine maleate salt;N-(4-Methoxyphenyl)methyl-N′,N′-dimethyl-N-(2-pyridinyl)-1,2-ethanediamine maleate salt;Pyrilamine maleate salt
- CAS NO.:59-33-6
- Empirical Formula: C21H27N3O5
- Molecular Weight: 401.46
- MDL number: MFCD00069333
- EINECS: 200-422-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
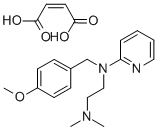
What is PYRILAMINE MALEATE?
Description
Mepyramine is a first generation antihistamine that acts as an inverse agonist at the histamine H1 receptor. It is reported to bind with high affinity to a Gq/11 protein-coupled form of the receptor and to promote a G protein-coupled inactive state of the H1 receptor that interferes with the Gq/11-mediated signaling of the endogenously expressed receptor, as well as to reduce G protein availability for other non-related receptors associated with this signaling pathway. Mepyramine has been shown to inhibit histamine-induced inositol phosphate production with a log EC50 value of -7.94.
Chemical properties
solid
The Uses of PYRILAMINE MALEATE
Pyrilamine maleate Selective inverse agonist for the H-1 histamine receptor. Pyrilamine Maleate is commonly utilized as a radioligand ([3H]Mepyramine) binding assay for the H1 receptor. And also used to exhibit blocking ability of KCNQ/M channels which is potentially related to an adverse effect seen in excess intake of antihistamines.
The Uses of PYRILAMINE MALEATE
Mepyramine maleate is used as bulk pharmaceutical (antihistaminic). Product Data Sheet
The Uses of PYRILAMINE MALEATE
A histamine H1 receptor antagonist
What are the applications of Application
Mepyramine maleate is a histamine H1 receptor antagonist
brand name
Histavet-P [Veterinary] (Schering-Plough Animal Health); Pymafed (HoechstRoussel).
General Description
White crystals or powder. Melting point 100-101°C. Bitter saline taste. An antihistaminic medicine.
General Description
Pyrilamine, 2-[4-methoxybenzyl[2-dimethylamino)ethyl]-amino]pyridine, is available as theacid maleate salt (1:1), which is a white crystalline powderwith a faint odor and a bitter, saline taste. The salt is solublein water (1:0.4) and freely soluble in alcohol. A 10% solutionhas a pH of approximately 5. At a pH of 7.5 or above,the free base begins to precipitate.
Pyrilamine differs structurally from tripelennamine byhaving a methoxy group in the para position of the benzylradical. It differs from its more toxic and less potent precursorphenbenzamine (Antergan) by having a 2-pyridyl groupon the nitrogen atom in place of a phenyl group.
Clinically, pyrilamine and tripelennamine are consideredamong the less potent antihistaminics. They are highly potent,however, in antagonizing histamine-induced contractionsof guinea pig ileum. Because of the pronounced localanesthetic action, the drug should not be chewed, but takenwith food.
Air & Water Reactions
Water soluble.
Reactivity Profile
PYRILAMINE MALEATE is an acidic salt of an amine. Usually does not react as either oxidizing agents or reducing agents but such behavior is not impossible. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
SYMPTOMS: Symptoms of exposure to PYRILAMINE MALEATE may include drowsiness, potentiation of the sedative effect of barbituates, fatigues, vertigo, incoordination, tremor, muscle weakness, dryness of the mouth and throat, tinnitus, pupillary dilation, blurred vision, urinary retention, impotence, epigastric and intestinal pain, anorexia, nausea, vomiting, diarrhea, excitation, euphoria, insomnia, nervousness, palpitation, tachycardia, hypotension, hypersensitivity reactions, respiratory depression, coma and delirium.
Fire Hazard
Flash point data for PYRILAMINE MALEATE are not available, however PYRILAMINE MALEATE is probably combustible.
Biological Activity
Selective inverse agonist for the H 1 receptor. Inhibits histamine induced inositol phosphate (InsP) production (log EC50 = -7.94) and intracellular calcium mobilization. Sequesters G q/11 protein, reducing its availability for other receptors associated with the same signaling pathway.
Biochem/physiol Actions
Pyrilamine maleate is a H1 histamine receptor antagonist. Pyrilamine maleate induces sleep in vertebrates. In Cassiopea, it induces concentration dependent dormancy.
Safety Profile
A human poison by ingestion. An experimental poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. An antihistamine. When heated to decomposition it emits toxic fumes of NOx.
Veterinary Drugs and Treatments
Antihistamines are used in veterinary medicine to reduce or help prevent histamine mediated adverse effects; predominantly used in horses.
storage
Room temperature
Properties of PYRILAMINE MALEATE
| Melting point: | 101-103°C |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, freely soluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | White |
| Water Solubility | Soluble in water at 100mg/ml |
| λmax | 310nm(EtOH)(lit.) |
| Merck | 14,7984 |
| Stability: | Stability Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 59-33-6(CAS DataBase Reference) |
| EPA Substance Registry System | Pyrilamine maleate (59-33-6) |
Safety information for PYRILAMINE MALEATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PYRILAMINE MALEATE
PYRILAMINE MALEATE manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
![[[(2-aminoethyl)amino]methyl]phenol](https://img.chemicalbook.in/CAS/GIF/53894-28-3.gif)
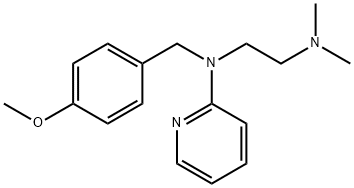
You may like
-
 Mepyramine maleate 59-33-6 99%View Details
Mepyramine maleate 59-33-6 99%View Details
59-33-6 -
 Mepyramine maleate 99%View Details
Mepyramine maleate 99%View Details
59-33-6 -
 PYRILAMINE MALEATE 95-99%View Details
PYRILAMINE MALEATE 95-99%View Details
59-33-6 -
 Pyrilamine Maleate CAS 59-33-6View Details
Pyrilamine Maleate CAS 59-33-6View Details
59-33-6 -
 Pyrilamine maleate CAS 59-33-6View Details
Pyrilamine maleate CAS 59-33-6View Details
59-33-6 -
 Pyrilamine maleate salt CAS 59-33-6View Details
Pyrilamine maleate salt CAS 59-33-6View Details
59-33-6 -
 Pyrilamine maleate CAS 59-33-6View Details
Pyrilamine maleate CAS 59-33-6View Details
59-33-6 -
 Mepyramine maleate CAS 59-33-6View Details
Mepyramine maleate CAS 59-33-6View Details
59-33-6
