Prucalopride
Synonym(s):4-Amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide
- CAS NO.:179474-81-8
- Empirical Formula: C18H26ClN3O3
- Molecular Weight: 367.87
- MDL number: MFCD09837787
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
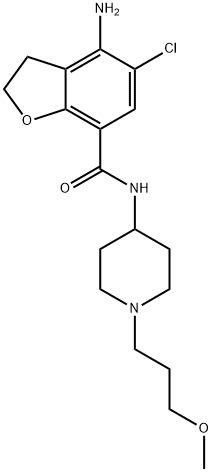
What is Prucalopride?
Absorption
Prucalopride is well absorbed and it reaches maximum plasma concentration of 3.79ng/ml with a tmax of 2.77 hours after initial administration. It presents an AUC of 96.5 mn.h/ml. The bioavailability of prucalopride is of over 90% and this bioavailability does not get influenced by the ingestion of food.
Toxicity
Prucalopride is well tolerated in doses reaching 10 times the recommended therapeutic dose and signs of overdose are thought to be stared by the presence of headaches, nausea and diarrhea. Carcinogenicity studies in mice indicated an increased incidence of mammary gland adenocarcinoma at a dose of 80 mg/kg/day. In rats, high doses were linked to increased incidence of benign adrenal pheochromocytoma, pituitary adenoma, pancreatic adenoma, hepatocellular adenoma and thyroid follicular tumors.
For genotoxicity, prucalopride showed only one weak positive result in one of the five bacterial strain reverse mutation test at high concentrations. As well, there is no evidence of adverse effects on fertility, even in high doses.
Description
Prucalopride is a potent and selective agonist of the serotonin (5-HT) receptor subtype 5-HT4 (Kis = 2.5 and 8 nM for human 5-HT4A and 5-HT4B, respectively). Prucalopride is greater than 250-fold selective for 5-HT4 over a panel of 53 overexpressed receptors, including 5-HT subtypes, but does bind to human dopamine D4 and sigma-1 (σ1) receptors and mouse 5-HT3 receptors (Kis = 2.3, 3.7, and 3.8 μM, respectively). It induces contractions in guinea pig colon with an EC50 value of 33 nM, an effect that is blocked by the 5-HT4 antagonist GR113808 but not the 5-HT2A and 5-HT3 antagonists ketanserin and granisetron , respectively. It also facilitates non-cholinergic contractions induced by electrical stimulation. In fasted dogs, oral administration of prucalopride increases colonic motility by inhibiting distal colon contractions (ED50 = 0.04 mg/kg), an effect that is blocked by pretreatment with the 5-HT4 antagonist GR125487. Prucalopride (5-10 mg/kg, s.c.) increases acetylcholine and histamine levels in the rat prefrontal cortex by 2.4-fold and 3-fold, respectively, and increases the power of hippocampal theta oscillations. Formulations containing prucalopride have been used in the treatment of chronic idiopathic constipation.
Indications
Prucalopride is indicated for the treatment of chronic idiopathic constipation (CIC) in adults.
CIC is one of the most common chronic functional gastrointestinal disorders worldwide. The diagnosis of this agent is very hard and it can be confirmed if the patient experience at least two of the following:
-Straining during more than 25% of the bowel movements.
-Lumpy or hard stools in 25% of the bowel movements.
-Sensation of incomplete evacuation in more than 25% of all bowel movements.
-Sensation of anorectal blockage or obstruction in more than 25% of the bowel movements.
-Manual maneuvers required in more than 25% of the bowel movements.
-Fewer than 3 bowel movements per week.
Background
Prucalopride is a dihydrobenzofurancarboxamide derivative from the benzofurane family that selectively stimulates 5-HT4 receptors and thus, it presents enterokinetic properties. The high selectivity of prucalopride allowed further development as it prevented the cardiac adverse reactions observed due to non-target effects of precedent therapies. Prucalopride was developed by Shire Development LLC and approved for use in Europe in 2009, in Canada on December 7, 2011 and by the FDA on December 17, 2018.
Definition
ChEBI: Prucalopride is a member of benzamides.
Biochem/physiol Actions
In healthy male individuals, prucalopride decreases the display of esophageal acid and promotes gastric emptying. It is effective, safe and shows good tolerance for the treatment of men with chronic constipation.
Pharmacokinetics
In animal studies, prucalopride induced a dose-dependent stimulation of contractile activity in the proximal colon and inhibition of the contractility in the distal colon. As well it has been shown that prucalopride stimulates and amplifies giant migratory contraction which is the high-amplitude type of contraction that initiates the urge to defecate. Thus, prucalopride not only accelerates the colonic transit but also accelerates gastric emptying and small bowel transit.
In supratherapeutic concentrations, prucalopride can be observed to interact with hERG potassium channels and L-type calcium channels.
In clinical trials, prucalopride showed to significantly increase the spontaneous bowel movements with a standardized mean difference of about 0.5 after the use of 1 mg when compared with the placebo group. In this studies as well, it was observed a numerical improvement in mean colonic transit time and a significant increase in spontaneous complete bowel movement without marked changes in the anorectal function.
In phase III clinical trials, 86% of the tested individuals opted to continue with the open-label study and based on Patients Assessments, 67% of the patients increase more than one point improvement in their satisfaction.
In the final set of clinical trials for approval, there was a significant increase in the number of patients that reached over 3 complete spontaneous bowel movements per week when compared with the placebo.
Enzyme inhibitor
This novel enterokinetic drug (FW = 367.87 g/mol; CAS 179474-81-8), also known by the tradename Resolor? and its systematic name, 4-amino-5- chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7- carboxamide, is a selective, high affinity serotonin 5-HT4 receptor agonist with that stimulates colonic mass movements, which provide the main propulsive force for defecation. It exhibits high affinity to both 5-HT4 receptor isoforms, with respective pKi values of 8.60 and 8.10 for the human 5-HT4A and 5-HT4B receptors. Based on 50 other binding assays,tonly the human D4 receptor (pKi = 5.63), the mouse 5-HT3 receptor (pKi = 5.41) and the human s1 (pKi = 5.43) shown measurable affinity, resulting in >290x selectivity for 5-HT4 receptors. Prucalopride also differs from other 5-HT4 agonists, such as tegaserod and cisapride, that interact with other receptors (5-HT1B/D and cardiac human ether-a-go-go K+ or hERG channel, respectively).
Metabolism
Prucalopride is not extensively metabolized in the body and does not interact with the enzymes of the family of the cytochrome P450 enzymes nor the P glycoprotein. The metabolism of prucalopride only represents 6% of the administered dose and the remaining 94% is found as the unchanged drug. From studies, it was reported the recovery of 8 metabolites being the major metabolite R107504 which is formed after the O-demethylation and oxidation of the resulting alcohol to a carboxylic acid.
Properties of Prucalopride
| Melting point: | 90.7° |
| Boiling point: | 481.4±45.0 °C(Predicted) |
| Density | 1.28 |
| storage temp. | 2-8°C |
| solubility | DMF: 20 mg/ml; DMF:PBS (pH 7.2) (1:9): 0.1 mg/ml; DMSO: 10 mg/ml; Ethanol: 10 mg/ml |
| pka | 13.65±0.20(Predicted) |
| form | powder |
| color | white to beige |
| InChI | InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) |
Safety information for Prucalopride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Prucalopride
| InChIKey | ZPMNHBXQOOVQJL-UHFFFAOYSA-N |
| SMILES | O1C2=C(C(NC3CCN(CCCOC)CC3)=O)C=C(Cl)C(N)=C2CC1 |
Prucalopride manufacturer
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
![4-amino-5-chloro-N-[1-(3-methoxypropyl) piperidin-4-yl]-2,3-dihydro-1- benzofuran-7-carboxamide monobutanedioate 179474-81-8 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-amino-5-chloro-N-[1-(3-methoxypropyl) piperidin-4-yl]-2,3-dihydro-1- benzofuran-7-carboxamide monobutanedioate 179474-81-8 98%View Details
4-amino-5-chloro-N-[1-(3-methoxypropyl) piperidin-4-yl]-2,3-dihydro-1- benzofuran-7-carboxamide monobutanedioate 179474-81-8 98%View Details
179474-81-8 -
 Prucalopride 99% (HPLC) CAS 179474-81-8View Details
Prucalopride 99% (HPLC) CAS 179474-81-8View Details
179474-81-8 -
 Prucalopride 98% CAS 179474-81-8View Details
Prucalopride 98% CAS 179474-81-8View Details
179474-81-8 -
 Prucalopride CAS 179474-81-8View Details
Prucalopride CAS 179474-81-8View Details
179474-81-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
