4-Aminosalicylic acid
Synonym(s):4-Amino-2-hydroxybenzoic acid;4-Aminosalicylic acid;PAS
- CAS NO.:65-49-6
- Empirical Formula: C7H7NO3
- Molecular Weight: 153.14
- MDL number: MFCD00007789
- EINECS: 200-613-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
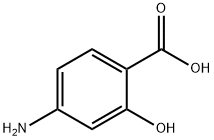
What is 4-Aminosalicylic acid?
Toxicity
LD50=4 gm/kg (orally in mice); LD50=3650 mg/kg (orally in rabbits)
Background
An antitubercular agent often administered in association with isoniazid. The sodium salt of the drug is better tolerated than the free acid.
Biological Activity
4-Aminosalicylic acid is an antimetabolite of p-aminobenzoic acid (PABA) that has antibacterial activity.It is active against streptomycin-sensitive and -resistant strains of M. tuberculosis (MICs = 0.78 and 0.39 μg/ml, respectively), an effect that can be reversed by PABA. 4-Aminosalicylic acid is an alternative substrate for mycobacterial dihydropteroate synthase (FolP1) and misincorporation into the folate pathway leads to accumulation of several folate-dependent metabolites including serine, homocysteine, dUMP, and AICAR, markers of folate pathway inhibition, in a concentration-dependent manner. It reverses manganese-induced increases in rat hippocampal levels of NOD-like receptor protein 3 (NLRP3), cleaved caspase-1, and phosphorylated p65, markers of NLRP3 inflammasome-dependent pyroptosis, when administered at a dose of 300 mg/kg. 4-Aminosalycilic acid is also a building block that has been used in the synthesis of luminescent lanthanide complexes. Formulations containing 4-aminosalicylic acid have been used in the treatment of tuberculosis.
Mechanism of action
p-aminosalicylic acid is thought to act as an antimetabolite interfering with the incorporation of p-aminobenzoic acid into folic acid. When coadministered with INH, PAS is found to reduce the acetylation of INH, itself being the substrate for acetylation, thus increasing the plasma levels of INH. This action may be especially valuable in patients who are rapid acetylators.
Pharmacokinetics
Aminosalicylic acid is an anti-mycobacterial agent used with other anti-tuberculosis drugs (most often isoniazid) for the treatment of all forms of active tuberculosis due to susceptible strains of tubercle bacilli. The two major considerations in the clinical pharmacology of aminosalicylic acid are the prompt production of a toxic inactive metabolite under acid conditions and the short serum half life of one hour for the free drug. Aminosalicylic acid is bacteriostatic against Mycobacterium tuberculosis (prevents the multiplying of bacteria without destroying them). It also inhibits the onset of bacterial resistance to streptomycin and isoniazid.
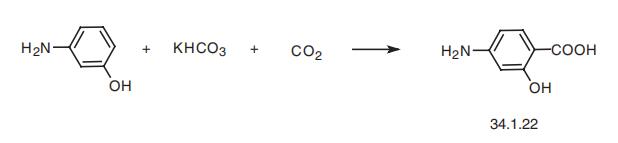
Synthesis
p-Aminosalicylic acid, 5-amino-2-hydroxybenzoic acid (34.1.22), is synthesized in a Kolbe reaction, which consists of direct interaction of m-aminophenol with potassium bicarbonate and carbon dioxide while heating at a moderate pressure of 5¨C10 atm.
Properties of 4-Aminosalicylic acid
| Melting point: | 135-145 °C (lit.) |
| Boiling point: | 276.03°C (rough estimate) |
| Density | 1.3585 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | 1.69g/l |
| form | Powder |
| pka | 3.25(at 25℃) |
| color | Colorless |
| PH | 3.5 (1g/l, H2O, 20℃) |
| Water Solubility | 2 g/L (20 ºC) |
| Merck | 14,477 |
| BRN | 473071 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 65-49-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Aminosalicylic acid(65-49-6) |
| EPA Substance Registry System | 4-Aminosalicylic acid (65-49-6) |
Safety information for 4-Aminosalicylic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 4-Aminosalicylic acid
4-Aminosalicylic acid manufacturer
Molsyns Research
Clickchem Research LLP
SETV ASRV LLP
ARRAKIS INDUSTRIES LLP
Biochemical and Synthetic Products PvtLtd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 65-49-6 4-Amino (Para Amino)Salicylic Acid (PAS Acid)- USP 98%View Details
65-49-6 4-Amino (Para Amino)Salicylic Acid (PAS Acid)- USP 98%View Details
65-49-6 -
 4-Aminosalicylic Acid 98%View Details
4-Aminosalicylic Acid 98%View Details -
 4-Aminosalicylic acid, 99% CAS 65-49-6View Details
4-Aminosalicylic acid, 99% CAS 65-49-6View Details
65-49-6 -
 65-49-6 SODIUM AMINOSALICYLATE 95-99%View Details
65-49-6 SODIUM AMINOSALICYLATE 95-99%View Details
65-49-6 -
 Schiff's reagent CASView Details
Schiff's reagent CASView Details -
 SCHIFFS REAGENT 99%View Details
SCHIFFS REAGENT 99%View Details -
 SCHIFF`S REAGENT CASView Details
SCHIFF`S REAGENT CASView Details -
 Schiff′s reagent CASView Details
Schiff′s reagent CASView Details
