Propafenone
- CAS NO.:54063-53-5
- Empirical Formula: C21H27NO3
- Molecular Weight: 341.44
- MDL number: MFCD00216020
- EINECS: 258-955-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
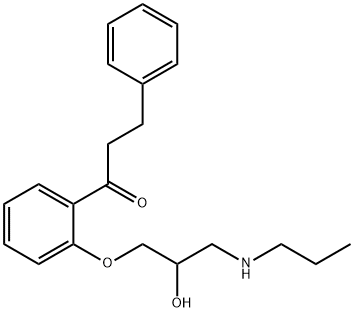
What is Propafenone?
Absorption
Nearly completely absorbed following oral administration (90%). Systemic bioavailability ranges from 5 to 50%, due to significant first-pass metabolism. This wide range in systemic bioavailability is related to two factors: presence of food (food increases bioavailability) and dosage (bioavailability is 3.4% for a 150-mg tablet compared to 10.6% for a 300-mg tablet).
Toxicity
Symptoms of propafenone overdose (usually most severe within the first 3 hours) may include convulsions (rarely), heartbeat irregularities, low blood pressure, and sleepiness.
The Uses of Propafenone
Cardiac depressant (anti-arrhythmic).
Indications
Used to prolong the time to recurrence of paroxysmal atrial fibrillation/flutter (PAF) associated with disabling symptoms in patients without structural heart disease. Also used for the treatment of life-threatening documented ventricular arrhythmias, such as sustained ventricular tachycardia.
Background
An antiarrhythmia agent that is particularly effective in ventricular arrhythmias. It also has weak beta-blocking activity. The drug is generally well tolerated.
Definition
ChEBI: An aromatic ketone that is 3-(propylamino)propane-1,2-diol in which the hydrogen of the primary hydroxy group is replaced by a 2-(3-phenylpropanoyl)phenyl group. It is a class 1C antiarrhythmic drug with local anesthetic effects, and is used as the hydroch oride salt in the management of supraventricular and ventricular arrhythmias.
brand name
Rythmol (Reliant);Arythmol;Nofenan;Nofenon;Nomorytmin;Normotrytmin (r) 10 mg;Prolekofen;Retmonorm;Ryhmonorma;Rythmole;Rytmonorm.
World Health Organization (WHO)
Propafenone, a membrane-stabilizing antiarrhythmic agent, was introduced into medicine in the mid 1980s. Shortly afterwards, its use became associated with cases of severe cardiac arrhythmias, which led to notable restrictions in the drug's indications in at least two countries. See also WHO comment for flecainide.
General Description
Propafenone, 2-[2'-hydroxy-3-(propylamino)propoxy]-3-phenylpropiophenone (Rythmol), a classIC antiarrhythmic drug, contains a chiral center and is marketedas the racemic mixture. Therapy with the racemicmixture of propafenone produces effects that can be attributedto both (S) and (R) enantiomers. Although (R) and (S)enantiomers exert similarNa+channel–blocking effects, the(S) enantiomer also produces aβ-adrenergic blockade. As aresult, the (S) enantiomer is reported to be 40-fold morepotent than the (R) enantiomer as an antiarrhythmic agent.The enantiomers also display stereoselective dispositioncharacteristics. The (R) enantiomer is cleared more quickly.Hepatic metabolism is polymorphic and determined genetically.Ten percent of Caucasians have a reduced capacity tohydroxylate the drug to form 5-hydroxypropafenone. Thispolymorphic metabolism accounts for the interindividualvariability in the relationships between dose and concentrationand, thus, variability in the pharmacodynamic effects ofthe drug. The 5-hydroxy metabolites of both enantiomersare as potent as the parent compound in blocking Na+channels.Propafenone also depresses the slow inward current ofCa2+ions.
Mechanism of action
Propafenone has not only pronounced class I effects but also class II (structure related) and class IV activities . Accordingly, it has a broad spectrum of activity against ventricular and supraventricular arrhythmias.
Pharmacokinetics
Propafenone is a Class 1C antiarrhythmic drug with local anesthetic effects, and a direct stabilizing action on myocardial membranes. It is used in the treatment of atrial and ventricular arrhythmias. It acts by inhibiting sodium channels to restrict the entry of sodium into cardiac cells resulting in reduced excitation. Propafenone has local anesthetic activity approximately equal to procaine.
Clinical Use
Propafenone has been used for acute termination orlong-term suppression of ventricular arrhythmias. It isbound in excess of 95% to 1-acid glycoprotein in theplasma. It is absorbed effectively, but bioavailability is estimatedto be less than 20% because of first-pass metabolism.Less than 1% is eliminated as unchanged drug. Therapywith propafenone may produce effects that can be attributedto both (S) and (R) enantiomers. Thus, the effects may bemodulated because of an enantiomer–enantiomer interactionwhen patients are treated with the racemate.
Side Effects
Concurrent administration of propafenone with
digoxin, warfarin, propranolol, or metoprolol increases
the serum concentrations of the latter four drugs.
Cimetidine slightly increases the propafenone serum
concentrations. Additive pharmacological effects can
occur when lidocaine, procainamide, and quinidine are
combined with propafenone.
As with other members of class IC, propafenone
may interact in an unfavorable way with other agents
that depress A-V nodal function, intraventricular conduction,
or myocardial contractility.
Overall, 21 to 32% of patients have adverse effects.
The most common are dizziness or light-headedness,
metallic taste, nausea, and vomiting; the most serious
are proarrhythmic events.
Metabolism
Metabolized primarily in the liver where it is rapidly and extensively metabolized to two active metabolites, 5-hydroxypropafenone and N-depropylpropafenone. These metabolites have antiarrhythmic activity comparable to propafenone but are present in concentrations less than 25% of propafenone concentrations.
Precautions
Propafenone is contraindicated in the presence of severe or uncontrolled congestive heart failure; cardiogenic shock; sinoatrial, A-V, and intraventricular disorders of conduction; and sinus node dysfunction, such as sick sinus syndrome. Other contraindications include severe bradycardia, hypotension, obstructive pulmonary disease, and hepatic and renal failure. Because of its weak β-blocking action, propafenone may cause possible dose-related bronchospasm.This problem is greatest in patients who are slow metabolizers.
Properties of Propafenone
| Boiling point: | 519.6±50.0 °C(Predicted) |
| Density | 1.096±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 68 mg/mL (199.16 mM);Ethanol: 41 mg/mL (120.08 mM) |
| pka | pKa 9.27 (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 759.9ug/L(22.5 ºC) |
| CAS DataBase Reference | 54063-53-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Propafenone(54063-53-5) |
Safety information for Propafenone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Propafenone
Propafenone manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 54063-53-5 Propafenone 98%View Details
54063-53-5 Propafenone 98%View Details
54063-53-5 -
 54063-53-5 99%View Details
54063-53-5 99%View Details
54063-53-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8