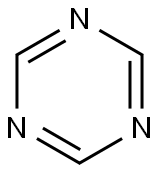
Prometryn
Synonym(s):2,4-Bis-(isopropylamino)-6-methylthio-1,3,5-triazine
- CAS NO.:7287-19-6
- Empirical Formula: C10H19N5S
- Molecular Weight: 241.36
- MDL number: MFCD00055396
- EINECS: 230-711-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
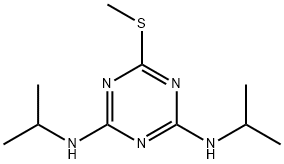
What is Prometryn?
Description
Prometryn can be used in Pre- and postemergence to Control Annual grasses, broadleaf weeds.
Chemical properties
Colorless crystalline solid.
The Uses of Prometryn
Prometryn is a methylthiotriazine herbicide used for the control of several annual grasses and broadleaf weeds. Prometryn works by inhibiting the electron transport in target broadleaves and grasses.
The Uses of Prometryn
Herbicide.
The Uses of Prometryn
Selective herbicide used to control many annual grasses and broad-leaved weeds in cotton, celery and peas.
Definition
ChEBI: A diamino-1,3,5-triazine that is N,N'-di(propan-2-yl)-1,3,5-triazine-2,4-diamine substituted by a methylsulfanediyl group at position 6.
Production Methods
Prometryne is made either by reacting propazine with methyl
mercaptan in the presence of one equivalent of NaOH or
by reacting 2-mercapto-4,6-bis(isopropylamino)-5-triazine
with a methylating agent in the presence of NaOH.
Prometryne is made either by reacting propazine with methyl
mercaptan in the presence of one equivalent of NaOH or
by reacting 2-mercapto-4,6-bis(isopropylamino)-5-triazine
with a methylating agent in the presence of NaOH.
General Description
Colorless crystals. Used as an herbicide.
Reactivity Profile
A triazine derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Flammability and Explosibility
Not classified
Agricultural Uses
Herbicide: Prometryn is used to control several annual grasses and broadleaf weeds in terrestrial food and feed crops. Its major uses are on cotton and celery, and is often used on dill and pigeon peas. Not approved for use in EU countries. Registered for use in the U.S.
Trade name
A-1114®; CAPAROL®; COTTON PRO®; G 34161®; GESAGARD®; MERCASIN®; MERCAZIN®; MERKAZIN®; POLISIN®; PRIMAPIN®; PRIMATOL-Q®; PROMET®; PROMETREX®; SELECTIN®; SELECTIN-50®; SELEKTIN®; SESAGARD®; SUPREND®; UVON®
Potential Exposure
Prometryn, a triazine herbicide, is used to control several annual grasses and broadleaf weeds in terrestrial food and feed crops. Among its major applica- tions are on cotton and celery and is often used on dill and pigeon peas.
Carcinogenicity
No carcinogenic activity was seen in either the rat or the mouse following lifetime feeding of up to 1500 or 3000 ppm, respectively.
Environmental Fate
Soil. In soil and plants, the methylthio group is oxidized. The proposed degradative
pathway is the formation of the corresponding sulfoxide and sulfone derivatives of prometryn followed by oxidation of the latter to forming hydroxypropazine (Kearney and
Kaufman, 1976). Cook and Hütter (1982) reported that bacterial cultures degraded prometryne to form the corresponding hydroxy derivative (hydroxyprometryne). 14C-Prometryn
was incubated in an organic soil for one year. It was observed that 57.4% of the bound
residues were comprised of prometryn (>50%), hydroxypropazine, mono-n-dealkylated prometryn, mono-N-dealkylated hydroxypropazine, a didealkylated compound [2-(methylthio)-4-amino-6-(isopropylamino)-s-triazine] and unidentified methanol soluble products (Khan and Hamilton, 1980; Khan, 1982). Hydroxyprometryn and ammeline were
reported as hydrolysis metabolites in soil (Somasundaram et al., 1991). The reported halflife in soil is 60 days (Jury et al., 1987).
Photolytic. When prometryn in aqueous solution was exposed to UV light for 3 hours,
the herbicide was completely converted to hydroxypropazine. Irradiation of soil suspensions containing prometryn was found to be more resistant to photodecomposition. About
75% of the applied amount was converted to hydroxypropazine after 72 hours of exposure
(Khan, 1982). The UV (λ = 253.7 nm) photolysis of prometryn in water, methanol, ethanol,
n-butanol and benzene, yielded 2-methylthio-4,6-bis(isoprop-ylamino)-s-triazine. At
wavelengths >300 nm, photodegradation was not observed (Pape and Zabik, 1970). Khan
and Gamble (1983) also studied the UV irradiation (λ = 253.7 nm) of prometryn in distilled
water and dissolved humic substances. In distilled water, 2-hydroxy-4,6-bis(isopropylamino)-s-triazine and 4,6-bis(isopropylamino)-s-triazine formed as major products.
In the presence of humic/fulvic acids, 4-amino-6-(isopropylamino)-s-triazine was also
formed (Khan and Gamble, 1983). Pelizzetti et al. (1990) studied the aqueous photocatalytic degradation of prometryn and other s-triazines (ppb level) using simulated sunlight
(λ >340 nm) and titanium dioxide as a photocatalyst. Prometryn rapidly degraded forming
cyanuric acid, nitrates, sulfates, the intermediate tentatively identified as 2,4-diamino-6-
hydroxy-N,N′-bis(1-methyl-ethyl)-1,3,5-triazine and other intermediate compounds similar to those found for atrazine. It was suggested that the appearance of sulfate ions was
due to the attack of the methylthio group at the number two position. Mineralization of
cyanuric acid to carbon dioxide was not observed (Pelizzetti et al., 1990).
Chemical/Physical. Mascolo et al. (1995) studied the reaction of prometryn (100 μM)
with sodium hypochlorite (10 mM) at 25°C and pH 7. Degradation followed the following
pathway: prometryn → 2,4-(N,N′-diisopropyl)diamino-6-methylsulfinyl-1,3,5-triazine →
2,4-(N,N′-diisopropyl)diamino-6-methylsulfonyl-1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6-methanesulfonate ester 1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6-
hydroxy-1,3,5-triazine.
Shipping
Triazine pesticides, solid, toxic, n.o.s. require a shipping label of “poisonous materials.” This material fall in DOT/UN Hazard Class 6.1.
Toxicity evaluation
Toxicity class II or III, slightly to moderately toxic (depending on formulation)
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recom- mendations for the disposal of pesticides and pesticide containers. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Prometryn
| Melting point: | 118-120°C |
| Boiling point: | 309.64°C (rough estimate) |
| Density | 1.157 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5500 (estimate) |
| Flash point: | 2 °C |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | 6.3g/L in organic solvents at 20 ℃ |
| form | neat |
| pka | 3.76±0.41(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 48.27mg/L(20 ºC) |
| Merck | 13,7881 |
| BRN | 613575 |
| CAS DataBase Reference | 7287-19-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3,5-Triazine-2,4-diamine, N,N'-bis(1-methylethyl)-6-(methylthio)-(7287-19-6) |
| EPA Substance Registry System | Prometryn (7287-19-6) |
Safety information for Prometryn
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Prometryn
| InChIKey | AAEVYOVXGOFMJO-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Prometryn CAS 7287-19-6View Details
Prometryn CAS 7287-19-6View Details
7287-19-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8