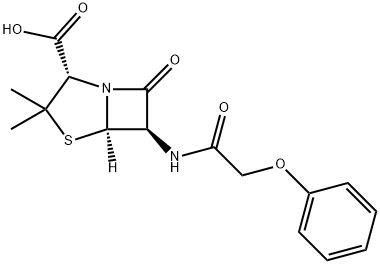
Procaine penicillin G
Synonym(s):Penicillin G procaine salt monohydrate
- CAS NO.:54-35-3
- Empirical Formula: C29H38N4O6S
- Molecular Weight: 570.7
- MDL number: MFCD00079285
- EINECS: 200-205-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
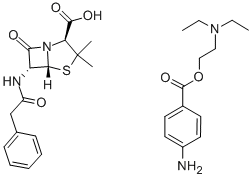
What is Procaine penicillin G?
Absorption
After intramuscular injection, it dissolves slowly at the site of injection, giving a plateau type of blood level at about 4 hours which falls slowly over a period of the next 15 to 20 hours.
Toxicity
Procaine benzylpenicillin is associated with the pain at the injection site, blood clotting problems, and seizures. Treatment targeted against syphilis is often associated with Jarisch-Herxheimer reaction. The main unwanted effects are hypersensitivity reactions caused by the degradation products of penicillin, which combine with host protein and become antigenic. Other common adverse effects include skin rashes, fever and delayed serum sickness. Rare but fatal anaphylactic shock may occur. Oral LD50 values in mouse and rat are > 2000 mg/kg. Overdosage can cause convulsions, paralysis and even death. Emesis and gastric lavage may be of value if begun within a few hours of injection. Excessive blood concentrations can be lowered by haemodialysis .
Chemical properties
White, fine crystals or powder; odorless; relatively stable to air and light; solutions dextroro- tatory. Sparingly soluble in water; slightly soluble in alcohol; fairly soluble in chloroform.
Originator
Duracillin,Lilly,US,1948
The Uses of Procaine penicillin G
Antibacterial.
Indications
For the treatment of a number of bacterial infections such as syphilis, anthrax, mouth infections, pneumonia and diphtheria.
Background
Procaine benzylpenicillin (INN), also known as procaine G penicillin, is an injectable antiobiotic. It is a poorly soluble salt form of penicillin which is a combination of naturally occuring benzylpenicillin (penicillin G) and the local anaesthetic agent procaine in equimolar amounts. Procaine benzylpenicillin is administered by deep intramuscular injection. It is slowly absorbed and hydrolyzed to benzylpenicillin. This drug is used where prolonged exposure to benzylpenicillin at a low concentration is required. This combination is aimed at reducing the pain and discomfort associated with a large intramuscular injection of penicillin. It is widely used in veterinary settings. Benzylpenicillin is active against a wide range of organisms and is the drug of first choice for many infections.
Manufacturing Process
There was added to 250 ml of a concentrated butyl acetate extract containing 74,000 units of the acid form of penicillin per ml, 50 ml of a butyl acetate solution containing 0.238 g per ml of procaine base. The solution was agitated for one hour. The precipitate which formed was very gummy and not in the form of discrete crystals. This precipitate was crystallized by scratching the side of the vessel and agitating further. After this treatment 18.25 g of crystalline procaine penicillin was obtained which assayed 1010 units per mg representing a yield of 99.6% of the activity contained in the concentrated extract.
brand name
Duracillin(Lilly); Pfizerpen (Pfizer).
Therapeutic Function
Antibacterial
General Description
The first widely used amine salt of penicillin G wasmade with procaine. Penicillin G procaine (Crysticillin,Duracillin, Wycillin) can be made readily from penicillin Gsodium by treatment with procaine hydrochloride. This saltis considerably less soluble in water than the alkali metalsalts, requiring about 250 mL to dissolve 1 g. Free penicillinis released only as the compound dissolves and dissociates.It has an activity of 1,009 units/mg. A large number ofpreparations for injection of penicillin G procaine are commerciallyavailable. Most of these are either suspensions inwater to which a suitable dispersing or suspending agent, a buffer, and a preservative have been added or suspensions inpeanut oil or sesame oil that have been gelled by theaddition of 2% aluminum monostearate. Some commercialproducts are mixtures of penicillin G potassium or sodiumwith penicillin G procaine; the water-soluble salt providesrapid development of a high plasma concentration of penicillin,and the insoluble salt prolongs the duration of effect.
Pharmacokinetics
It is an antibiotic against penicillin-susceptible microorganisms with bactericidal effect. Like all penicillins, procaine benzylpenicillin interferes with the synthesis of the bacterial cell wall peptidoglycan. It acts through the inhibition of biosynthesis of cell-wall peptidoglycan, rendering the cell wall osmotically unstable. It is part of the penicillin and beta lactam family of antibacterial drugs.
Safety Profile
Moderately toxic by intraperitoneal and intramuscular routes. Slightly toxic by ingestion and subcutaneous routes. When heated to decomposition it emits toxic vapors of NOx, and SOx.
Metabolism
Procaine is rapidly hydrolyzed by plasma esterases to nontoxic metabolites.
Properties of Procaine penicillin G
| Melting point: | 129-130 °C |
| Density | 1.1335 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO: 100 mg/mL (175.22 mM);Ethanol: 20 mg/mL (35.04 mM) |
| Water Solubility | Water: 10 mg/mL (17.52 mM) |
| CAS DataBase Reference | 54-35-3 |
| EPA Substance Registry System | 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]- (2S,5R,6R)-, compd. with 2-(diethylamino)ethyl 4-aminobenzoate (1:1) (54-35-3) |
Safety information for Procaine penicillin G
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Procaine penicillin G
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
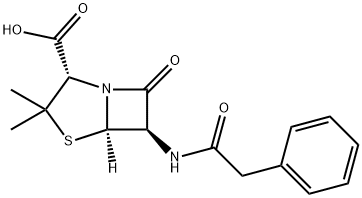
![4-AMINO-N-[2-(DIETHYLAMINO)ETHYL]BENZAMIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure1/GIF/CB1211904.gif)


You may like
-
 Procaine penicillin G 97% (HPLC) CAS 54-35-3View Details
Procaine penicillin G 97% (HPLC) CAS 54-35-3View Details
54-35-3 -
 Procaine Penicillin G CAS 54-35-3View Details
Procaine Penicillin G CAS 54-35-3View Details
54-35-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
