Penicillin G
Synonym(s):(2S,5R,6R)-3,3-Dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;Benzylpenicillin
- CAS NO.:61-33-6
- Empirical Formula: C16H18N2O4S
- Molecular Weight: 334.39
- MDL number: MFCD00069665
- EINECS: 200-506-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
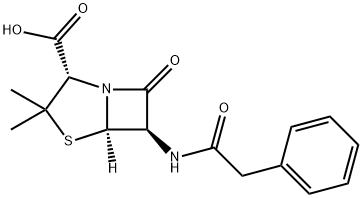
What is Penicillin G?
Absorption
Rapidly absorbed following both intramuscular and subcutaneous injection. Initial blood levels following parenteral administration are high but transient. Oral absorption in fasting, healthy humans is only about 15-30% as it is very susceptible to acid-catalyzed hydrolysis.
Toxicity
Oral LD50 in rat is 8900 mg/kg . Neurological adverse reactions, including convulsions, may occur with the attainment of high CSF levels of beta-lactams. Neutropenia can occur if high doses are administered consistently for over 2 weeks.
Description
Penicillin was the first natural antibiotic used to treat bacterial infections and continues to be one of the most important antibiotics.The name comes from the fungus genus Penicillium from which it was isolated. Penicillus is Latin for brush and refers to the brushlike appearance of filamentous Penicillium species.Species of this genus are quite common and appear as the bluish-green mold that appears on aged bread, fruit, and cheese. The term penicillin is a generic term that refers to a number of antibiotic compounds with the same basic structure. Therefore it is more appropriate to speak of penicillins than of penicillin.
The general penicillin structure consists of a β-lactam ring and thiazolidine ring fused together with a peptide bonded to a variable R group. Penicillin belongs to a group of compounds called β-lactam antibiotics. This in turn inhibits the formation of peptidoglycan cross-links in bacteria cell walls.
Description
The “wonder drug” penicillin is actually a group of drugs that have different functional groups on one of the side chains on the bicyclic core. In 1928, Scottish biologist Alexander Fleming isolated the first specific form of penicillin from Penicillium fungi; the compound is known variously as benzylpenicillin, penicillin G, or benzylpenicillinic acid. For this achievement, Fleming shared the 1945 Nobel Prize in Physiology or Medicine.
The free acid benzylpenicillin is only sparingly soluble in water; but even worse, it is inactivated by water. Thus, the articles of commerce are its sodium and potassium salts, which are much more water-soluble and stable in solution.
By the beginning of World War II, penicillin had been developed into antibacterial drugs that could be mass-produced. At the time of the Normandy invasion in 1944, more than 2 million doses had been made. Penicillin may have saved as many as 100,000 lives during the war.
Much more recently, it was discovered that some soil bacteria readily consume penicillin and other β-lactam antibiotics. Biologist Gautam Dantas and his team at Washington University (St. Louis) hypothesized that bacteria engineered to maximize β-lactam consumption capacity could be used to remediate antibiotic-contaminated soils.
The researchers created a strain of Escherichia coli that uses penicillin as its only carbon source. The downside, however, is that other organisms might also acquire the degradation genes and become resistant to penicillin.
History
The discovery of penicillin is generally credited to Alexander Fleming (1881 1955) in 1928, but the development of penicillin as an antibiotic took place sporadically over the last decades of the 19th century and first half of the 20th century. Mass production of penicillin by U.S.firms started in 1943,and it was used immediately to treat wounded soldiers. Penicillin reduced suff ering, prevented amputations,cured pneumonia,and saved thousands of lives during the war and was hailed as a miracle drug. The United States increased production throughout the war years and after the war widespread civilian use commenced. Fleming, Florey,and Chain shared the Nobel Prize in physiology or medicine in 1945 for their work on penicillin.
The Uses of Penicillin G
Antibacterial.
The Uses of Penicillin G
Benzylpenicillin is the drug of choice for infections caused by sensitive organisms. This includes streptococci infections (except enterococci), gonococci, and meningococci that do not produce beta-lactam anaerobes. Benzylpenicillin is used for croupous and focal pneumonia, skin infections, soft tissue and mucous membranes, periotonitis, cystisis, syphilis, diphtheria, and other infectious diseases. Synonyms of this drug are megacillin,
The Uses of Penicillin G
Penicillin is an antimicrobial agent.
Background
Benzylpenicillin (Penicillin G) is narrow spectrum antibiotic used to treat infections caused by susceptible bacteria. It is a natural penicillin antibiotic that is administered intravenously or intramuscularly due to poor oral absorption. Penicillin G may also be used in some cases as prophylaxis against susceptible organisms.
Natural penicillins are considered the drugs of choice for several infections caused by susceptible gram positive aerobic organisms, such as Streptococcus pneumoniae, groups A, B, C and G streptococci, nonenterococcal group D streptococci, viridans group streptococci, and non-penicillinase producing staphylococcus. Aminoglycosides may be added for synergy against group B streptococcus (S. agalactiae), S. viridans, and Enterococcus faecalis. The natural penicillins may also be used as first or second line agents against susceptible gram positive aerobic bacilli such as Bacillus anthracis, Corynebacterium diphtheriae, and Erysipelothrix rhusiopathiae. Natural penicillins have limited activity against gram negative organisms; however, they may be used in some cases to treat infections caused by Neisseria meningitidis and Pasteurella. They are not generally used to treat anaerobic infections. Resistance patterns, susceptibility and treatment guidelines vary across regions.
Indications
For use in the treatment of severe infections caused by penicillin G-susceptible microorganisms when rapid and high penicillin levels are required such as in the treatment of septicemia, meningitis, pericarditis, endocarditis and severe pneumonia.
Indications
Benzylpenicillin or penicillin G has a narrow antimicrobial spectrum. It is active with respect to Gram-positive bacteria (staphylococcus, streptococcus, and pneumococci), causative agent of diphtheria, and anthrax bacillus. Gram-negative bacteria are resistant to it. Benzylpenicillin is broken down by stomach acid and destroyed by staphylococcus penicillinase.
Definition
ChEBI: Benzylpenicillin is a penicillin in which the substituent at position 6 of the penam ring is a phenylacetamido group. It has a role as an antibacterial drug, an epitope and a drug allergen. It is a penicillin allergen and a penicillin. It is a conjugate acid of a benzylpenicillin(1-).
brand name
Pentids (Apothecon); Pfizerpen (Pfizer).
Antimicrobial activity
It has intrinsic activity against almost all Grampositive
pathogens, but is no longer effective against most
staphylococci. Most species of streptococci are susceptible,
including group B streptococci, an important cause of neonatal
infections. Enterococci are more resistant than streptococci.
Other susceptible Gram-positive organisms include
non-β-lactamase-producing Bacillus anthracis and Listeria monocytogenes.
The spirochetes Borrelia burgdorferi and
Treponema pallidum are also susceptible.
The aerobic Gram-negative cocci Neisseria gonorrhoeae
and N. meningitidis were initially highly susceptible to benzylpenicillin,
but β-lactamase-producing strains of gonococci
are now common. H. influenzae and Moraxella catarrhalis
are usually resistant due to β-lactamase production. The
Enterobacteriaceae and most other aerobic Gram-negative
bacilli are resistant, as a result of β-lactamase production
or the impermeability of the bacterial cell wall. Other resistant
organisms include mycobacteria, mycoplasmas, Nocardia
spp., rickettsiae and chlamydiae.
Anaerobic Gram-positive cocci are susceptible in
the absence of β-lactamase production. Most clostridia
strains are susceptible, but resistance can be observed.
Anaerobic Gram-negative bacilli vary in their sensitivity:
the Bacteroides fragilis group is resistant as the result of
β-lactamase action, but many Prevotella and Fusobacterium
spp. are susceptible.
Benzylpenicillin exhibits concentration-dependent bactericidal
activity against growing organisms. Killing of highly susceptible
Gram-positive cocci seldom proceeds to eradication,
with measurable numbers of survivors (‘persisters’), which
are fully susceptible on retesting. Some strains of streptococci
(including group B) and pneumococci show very large numbers
of persisters, resulting in a large difference between the
minimum inhibitory concentration (MIC) and the minimum
bactericidal concentration (MBC), a phenomenon known as
‘tolerance’.
Combination with aminoglycosides results in pronounced
bactericidal synergy.
Acquired resistance
Staph. aureus was originally highly susceptible to benzylpenicillin,
but at least 85–90% of clinical isolates are now
β-lactamase-producing strains. Most clinical isolates of coagulase-
negative staphylococci are also resistant. β-Lactamaseproducing
strains of E. faecalis produce an enzyme identical to
staphylococcal penicillinases, but these strains are increasingly
uncommon. The emergence of penicillin-resistant staphylococci,
enterococci and pneumococci, due to the acquisition
of mosaic PBPs with decreased binding affinity for penicillin,
has been described worldwide. Most strains of penicillin-
resistant Gram-positive clinical isolates also demonstrate
reduced susceptibility to other β-lactam agents but, in rare
cases, cross-resistance is not seen for all cephalosporins.
Strains of N. gonorrhoeae for which the MIC of benzylpenicillin
increased from 0.06 mg/L to >2 mg/L appeared in the
1970s, as the result of the production of modified PBPs with
reduced affinity for β-lactam antibiotics; fully resistant strains
producing TEM-1 β-lactamase also emerged. Currently penicillin
resistance occurs in more than 60% of isolates in some
parts of the world.
Contact allergens
Benzyl penicillin is actually used only intravenously. It was formerly a frequent cause of contact allergy in health care workers. Facial contact dermatitis was recently reported in a nurse.
Pharmacokinetics
Penicillin G is a penicillin beta-lactam antibiotic used in the treatment of bacterial infections caused by susceptible, usually gram-positive, organisms. The name "penicillin" can either refer to several variants of penicillin available, or to the group of antibiotics derived from the penicillins. Penicillin G has in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria. The bactericidal activity of penicillin G results from the inhibition of cell wall synthesis and is mediated through penicillin G binding to penicillin binding proteins (PBPs). Penicillin G is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended spectrum beta-lactamases.
Pharmacokinetics
Oral absorption: 2–25%
Cmax 0.6 g intramuscular: 12 mg/L
3 g intravenous (3–5 min): 400 mg/L
Plasma half-life: 0.5 h
Volume of distribution: 0.2–0.7 L/kg
Plasma protein binding: 60%
Absorption
Benzylpenicillin is unstable in acid and destroyed in the stomach.
As a result, plasma concentrations obtained after oral
administration are variable, and are depressed by administration
with food. It is absorbed from serous cavities, joints and
the subarachnoid space. It is not absorbed following applications
to the skin, which should be avoided because of the
likelihood
of sensitization.
Distribution
The drug is widely distributed in most tissues and body fluids.
Highest levels are found in the kidney, with lower levels in the
liver, skin and intestines. Low concentrations appear in saliva
and in maternal milk. It does not enter uninflamed bone or
the CSF. Its entry is limited by its low pKa (2.6), which results
in its almost complete ionization and very low lipid–water partition
coefficient at pH 7.4. When the meninges are inflamed,
the concentrations obtained in CSF are around 5% of the
plasma level. In uremia, accumulated organic acids may enter the CSF and compete for transport of penicillin, causing the
concentration to reach convulsive levels.
It diffuses into wound exudates and experimental transudates
when the serum level is high, and enters glandular
secretions and the fetal circulation, whence it is excreted in
increased concentrations into the amniotic fluid.
Metabolism and excretion
About 40% is metabolized in the liver, mainly to penicilloic
acid. After oral dosing, unabsorbed drug is largely degraded
by colonic bacteria and little activity remains in the feces.
Concentrations 2–4 times those of the plasma are found in
bile, but 60–90% is excreted in the urine, largely in the first
hour. Probenecid causes a doubling of the peak concentration
and prolongation of the plasma half-life. Other drugs, including
aspirin, sulfonamides and some non-steroidal anti-inflammatory
drugs and diuretics, may prolong the half-life.
Clinical Use
Serious infections caused by streptococci (including Str. pneumoniae)
other than meningitis caused by penicillin-resistant pneumococci
Serious infections caused by susceptible strains of staphylococci
Meningococcal septicemia and meningitis
Gonococcal infections caused by susceptible strains
Syphilis (including neurosyphilis) and other spirochetal infections
Anthrax
Actinomycosis
Clostridial infections
Diphtheria (adjunctive therapy to antitoxin and for prevention of carrier state)
Infections with other susceptible organisms, including Listeria
monocytogenes, Pasteurella multocida, Erysipelothrix insidiosa and
Fusobacterium
Clinical Use
For years, the most popular penicillin has been penicillin G,or benzylpenicillin. In fact, with the exception of patients allergicto it, penicillin G remains the agent of choice for thetreatment of more different kinds of bacterial infection thanany other antibiotic. It was first made available as the watersolublesalts of potassium, sodium, and calcium. These saltsof penicillin are inactivated by the gastric juice and are noteffective when administered orally unless antacids, such ascalcium carbonate, aluminum hydroxide, and magnesiumtrisilicate; or a strong buffer, such as sodium citrate, isadded. Also, because penicillin is absorbed poorly from the intestinal tract, oral doses must be very large, about fivetimes the amount necessary with parenteral administration.Only after the production of penicillin had increased enoughto make low-priced penicillin available did the oral dosageforms become popular. The water-soluble potassium andsodium salts are used orally and parenterally to achieve highplasma concentrations of penicillin G rapidly. The morewater-soluble potassium salt usually is preferred when largedoses are required. Situations in which hyperkalemia is adanger, however, as in renal failure, require use of thesodium salt; the potassium salt is preferred for patients onsalt-free diets or with congestive heart conditions.
Side Effects
Benzylpenicillin has low toxicity, except for the nervous system
(into which it does not normally penetrate), where it is
one of the most active convulsants among the β-lactam agents.
Excessively high intravenous doses may induce convulsions
and intrathecal doses should never exceed 12 mg (20 000
units) in adults or 3 mg (5000 units) in a child as a single daily
dose. Inadvertent intravascular administration, especially
direct injection into arteries, can cause serious neurotoxic
damage, including hyperreflexia, myoclonic twitches, seizures
and coma.
Massive intravenous doses of the sodium or potassium
salts can lead to severe or fatal electrolyte disturbances. In
patients treated with large doses of the potassium salt (60 g
or more per day), hyponatremia, hyperkalemia and metabolic
acidosis can develop.
Thrombocytopenia and platelet dysfunction resulting in
coagulopathy and involving several different mechanisms have
been described. Neutropenia associated with fever and allergic
rash appears to be related to total dose, usually in excess of
90 g. Although it can be very severe, in most patients recovery
occurs within a few days of withdrawal of treatment.
Large doses (24 g [40 megaunits] per day intravenously),
or smaller doses given to patients with impaired renal function,
may interfere with platelet function.
The most dramatic untoward response is anaphylactic
shock due to allergy . In addition to the generalized
allergic reactions, particular organs may be damaged by a
variety of immunological mechanisms. Hemolytic anemia
occurs only in patients who have been treated previously
with penicillin, and again receive a prolonged course of large
doses (commonly 12 g per day). Reversible hemolysis is due
to the action of anti-penicillin immunoglobulin G (IgG) on
cells that have absorbed the antibiotic. Nephritis, resulting
in dysuria, pyuria, proteinuria, azotemia and histological evidence of nephritis of allergic origin, is only rarely seen, usually
in patients receiving large doses (12–36 g [20–60 megaunits]
per day).
Safety Profile
Poison by ingestion, intravenous,intracerebral, intraspinal, subcutaneous, and possibly otherroutes. Human (child) systemic effects by parenteral route:changes in cochlear (inner ear) structure or function,convulsions, and dyspnea. Questionable carcin
Drug interactions
Potentially hazardous interactions with other drugs
Reduced excretion of methotrexate.
Metabolism
About 16-30% of an intramuscular dose is metabolized to penicilloic acid, an inactive metabolite. Small amounts of 6-aminopenicillanic acid have been recovered in the urine of patients on penicillin G. A small percentage of the drug appears to be hydroxylated into one or more active metabolites, which are also excreted via urine.
Metabolism
Benzylpenicillin is metabolised to a limited extent and the penicilloic acid derivative has been recovered in the urine. Benzylpenicillin is rapidly excreted in the urine; about 20% of an oral dose appears unchanged in the urine; about 60-90% of an IM dose of benzylpenicillin undergoes renal elimination, 10% by glomerular filtration and 90% by tubular secretion, mainly within the first hour. Significant concentrations occur in bile, but in patients with normal renal function only small amounts are excreted via the bile. Renal tubular secretion is inhibited by probenecid, which can be given to increase plasma-penicillin concentrations and prolong half-life.
Properties of Penicillin G
| Melting point: | 82-83 °C |
| Boiling point: | 663.3±55.0 °C(Predicted) |
| alpha | D +282° (ethanol) |
| Density | 1.2729 (rough estimate) |
| refractive index | 1.6930 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 100 mg/mL |
| form | powder |
| appearance | white crystals or powder |
| pka | 2.45±0.50(Predicted) |
| color | Crystals |
| Water Solubility | 2.675g/L(25 ºC) |
| CAS DataBase Reference | 61-33-6(CAS DataBase Reference) |
| EPA Substance Registry System | 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]- (2S,5R,6R)- (61-33-6) |
Safety information for Penicillin G
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Penicillin G
Penicillin G manufacturer
A.B.ENTERPRISES
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 61-33-6 Benzylpenicillin 98%View Details
61-33-6 Benzylpenicillin 98%View Details
61-33-6 -
 Benzylpenicillin 61-33-6 99%View Details
Benzylpenicillin 61-33-6 99%View Details
61-33-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8