Benzathine benzylpenicillin
Synonym(s):Penicillin G benzathine tetrahydrate
- CAS NO.:1538-09-6
- Empirical Formula: C48H56N6O8S2
- Molecular Weight: 909.12
- MDL number: MFCD00079284
- EINECS: 216-260-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
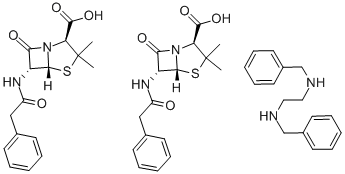
What is Benzathine benzylpenicillin?
Chemical properties
White or almost white powder.
Originator
Bicillin,Wyeth,US,1951
The Uses of Benzathine benzylpenicillin
Benzathine benzylpenicillin is used as a Penicillin antibacterial.
The Uses of Benzathine benzylpenicillin
The benzathine salt of penicillin G, an antibiotic used in the treatment of various bacterial infections, including Staphylococcus aureus.
What are the applications of Application
Penicillin G benzathine salt is a preparation of Penicillin G
Manufacturing Process
Ethylenediamine (15 g, 0.25 mol) was added dropwise to 100 ml 98-100% formic acid in a two-necked 500 ml flask, fitted with an addition tube and reflux condenser with drying tube, cooled in an ice-bath. After complete addition of the base, 53 g of benzaldehyde (0.5 mol) was added in one lot. The ice-bath was removed and the flask was heated to the refluxing temperature. The initial rate of carbon dioxide evolution was too rapid to measure. After twenty minutes, the rate was circa 100 ml per minute and decreased rapidly to 8 ml per minute in one hour. Heating at reflux was continued for 35 hours.
Following the refluxing most of the excess formic acid was removed under reduced pressure. Hydrochloric acid (200 ml 6 N) was added to the viscous amber residue and heated under reflux, After 15 minutes, bumping necessitated cooling and filtering to remove crystalline dihydrochloride, which after washing with isopropanol was dried, MP circa 300°C. The mother liquors were refluxed one hour and cooled, obtaining an additional amount of product, MP circa 300°C. The filtrate was concentrated in vacuo to 100 ml, cooled and made alkaline with 40% NaOH. The supernatant oil was extracted with ether, dried, and fractionated from a stillpot packed with glass wool and heated in a sand-bath at 320°C. The first fraction at 106°C at 0.6-0.7 mm was Nbenzylethylenediamine (dipicrate, MP 222°C). The N,N'dibenzylethylenediamine was collected at 177°C to 206°C at 0.6-1.0 mm as a colorless liquid.
To a solution of 60 g of sodium penicillin G in 800 cc of distilled water cooled to 0°C to 4°C in an ice-bath, a solution of 35 g of N,N'dibenzylethylenediamine diacetate in 200 cc of distilled water is added dropwise with stirring. The thick slurry is filtered with suction, washed twice with 100 cc of cold water, dried by suction and spread out in a thin layer for completion of drying. The product weighed 80 g.
The air-dried powder has a broad melting point, sintering at 100°C, melting above 110°C to a cloudy liquid becoming clear at 135°C.
Therapeutic Function
Antibacterial
Clinical Use
Since penicillin G benzathine, N,N'-dibenzylethylenediaminedipenicillin G (Bicillin, Permapen), is the salt of a diamine,2 moles of penicillin are available from each molecule. It isvery insoluble in water, requiring about 3,000 mL to dissolve1 g. This property gives the compound great stabilityand prolonged duration of effect. At the pH of gastric juice,it is quite stable, and food intake does not interfere with itsabsorption. It is available in tablet form and in several parenteralpreparations. The activity of penicillin G benzathineis equivalent to 1,211 units/mg.Several other amines have been used to make penicillinsalts, and research is continuing on this subject. Otheramines that have been used include 2-chloroprocaine; L-Nmethyl-1,2-diphenyl-2-hydroxyethylamine (L-ephenamine);dibenzylamine; tripelennamine (Pyribenzamine); and N,N'-bis-(dehydroabietyl)ethylenediamine (hydrabamine).
Safety Profile
Moderately toxic by ingestion andintraperitoneal routes. Experimental reproductive effects.When heated to decomposition it emits very toxic fumesof NOx and SOx. See other penicillin entries.
Properties of Benzathine benzylpenicillin
| Melting point: | 123-124° |
| alpha | D25 +206° (c = 0.105 in formamide) |
| solubility | Very slightly soluble in water, freely soluble in dimethylformamide and in formamide, slightly soluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | Crystals |
| CAS DataBase Reference | 1538-09-6 |
| EPA Substance Registry System | 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]- (2S,5R,6R)-, compd. with N,N'-bis(phenylmethyl)-1,2-ethanediamine (2:1) (1538-09-6) |
Safety information for Benzathine benzylpenicillin
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P284:Wear respiratory protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Benzathine benzylpenicillin
Benzathine benzylpenicillin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Benzathine penicillin 99%View Details
Benzathine penicillin 99%View Details -
 1538-09-6 98%View Details
1538-09-6 98%View Details
1538-09-6 -
 Penicillin G benzathine salt with 1% Lecithin 96.0-102.0% CAS 1538-09-6View Details
Penicillin G benzathine salt with 1% Lecithin 96.0-102.0% CAS 1538-09-6View Details
1538-09-6 -
 Penicillin G benzathine salt with 0.2% Polysorbate 80 98% (HPLC) CAS 1538-09-6View Details
Penicillin G benzathine salt with 0.2% Polysorbate 80 98% (HPLC) CAS 1538-09-6View Details
1538-09-6 -
 Benzathine benzylpenicillin CAS 1538-09-6View Details
Benzathine benzylpenicillin CAS 1538-09-6View Details
1538-09-6 -
 Benzathine benzylpenicillin CASView Details
Benzathine benzylpenicillin CASView Details -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1