Penicillin G potassium salt
Synonym(s):Benzylpenicillin potassium salt;Penicillin G potassium salt
- CAS NO.:113-98-4
- Empirical Formula: C16H17KN2O4S
- Molecular Weight: 372.48
- MDL number: MFCD00036193
- EINECS: 204-038-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
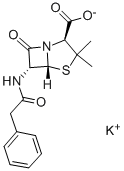
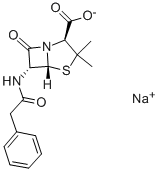
What is Penicillin G potassium salt?
Description
Benzylpenicillin potassiumwas the first crystalline penicillin produced on an industrial scale , . It is produced in the pure state by the addition of phenylacetate to a culture of Penicillium chrysogenum. The crystalline benzylpenicillin potassium contains 1598 U/mg.
Chemical properties
Penicillin G potassium salt is also known as Potassium benzylpenicillin, it is white crystalline powder, odorless or slightly specific odor, hygroscopic. Soluble in water, physiological saline, glucose solution. The aqueous solution of it is easy to fail when placed at room temperature, and it will fail rapidly in the presence of acid, alkali, oxidant, etc. Penicillin G potassium salt has good antibacterial effect on Streptococcus such as Streptococcus hemolyticus, Streptococcus pneumoniae and Staphylococcus without penicillinase.
The Uses of Penicillin G potassium salt
Penicillin G is a narrow spectrum antibiotic derived from Streptococcus pneumoniae. Penicillin G potassium salt is used as a cell culture additive as an antibiotics. Use to inhibit the synthesis of bacterial cell walls by inhibition of the cell wall peptidoglycan chain cross-lining. It is the drug of choice for groups A, B, C and G streptococci, nonenterococcal group D streptococci, viridians group streptococci, and non-penicillinase producing staphylococcus. The potassium salt has been used to study murosomes of staphylococci and the penicillin-induced lysis of Streptococcus mutans.
What are the applications of Application
Penicillin G potassium salt is a β-lactam antibiotic
What are the applications of Application
Penicillin G potassium salt, cell culture grade is a cell culture grade β-lactam antibiotic
Definition
ChEBI: Penicillin G potassium salt is organic potassium salt of benzylpenicillin. It is an antibiotic substance produced by Penicillium sp. Antibacterial. It contains a benzylpenicillin(1-).
Preparation
Penicillin G potassium salt is a highly effective and low toxicity antibiotic, which is obtained by salt formation of Chlorpheniramine maleate and maleic acid.
brand name
Pentids (Apothecon); Pfizerpen (Pfizer).
General Description
Penicillin G Potassium is the potassium salt form of penicillin G, a broad-spectrum penicillin antibiotic. Penicillin G potassium binds to penicillin binding proteins (PBP), the enzymes that catalyze the synthesis of peptidoglycan, which is a critical component of the bacterial cell wall. This leads to the interruption of cell wall synthesis, consequently leading to bacterial cell growth inhibition and cell lysis.
Biochem/physiol Actions
Mode of Action: Penicillin G acts by inhibiting cell wall synthesis through binding to penicillin binding proteins (PBPs), inhibiting peptidoglycan chain cross-linking.
Antimicrobial spectrum: This product is active against gram-positive and gram-negative bacteria.
Safety Profile
Poison by intracerebral andintravenous routes. Moderately toxic by intraperitonealroute. Mutation data reported. See other penicillin entries.When heated to decomposition it emits toxic fumes ofNOx and SOx.
Properties of Penicillin G potassium salt
| Melting point: | 214-217 C |
| alpha | D22 +285° (c = 0.748 in water) |
| refractive index | 294 ° (C=1, H2O) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | H2O: 100 mg/mL |
| form | powder |
| color | Needles from butanol (aq) |
| PH | pH (10g/L, 25℃) : 5.0~7.5 |
| Water Solubility | Soluble in water (100 mg/ml), methanol, ethanol (sparingly), and alcohol. Insoluble in chloroform. |
| Merck | 14,7094 |
| BRN | 3832841 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Penicillin G Potassium (113-98-4) |
Safety information for Penicillin G potassium salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Penicillin G potassium salt
| InChIKey | IYNDLOXRXUOGIU-LQDWTQKMSA-M |
| SMILES | N12C([C@@H](NC(=O)CC3=CC=CC=C3)[C@@]1([H])SC(C)(C)[C@@H]2C([O-])=O)=O.[K+] |&1:2,13,19,r| |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 113-98-4 Penicillin G potassium 98%View Details
113-98-4 Penicillin G potassium 98%View Details
113-98-4 -
 Penicillin G Potassium Salt CAS 113-98-4View Details
Penicillin G Potassium Salt CAS 113-98-4View Details
113-98-4 -
 Benzylpenicillin potassium CAS 113-98-4View Details
Benzylpenicillin potassium CAS 113-98-4View Details
113-98-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
