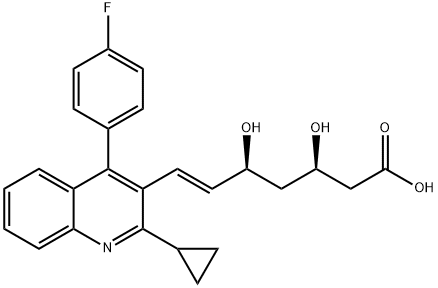
Pravastatin
- CAS NO.:81093-37-0
- Empirical Formula: C23H36O7
- Molecular Weight: 424.53
- MDL number: MFCD00869375
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
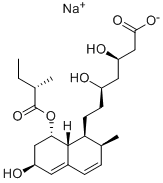
What is Pravastatin?
Absorption
Pravastatin is absorbed 60-90 min after oral administration and it presents a low bioavailability of 17%. This low bioavailability can be presented due to the polar nature of pravastatin which produces a high range of first-pass metabolism and incomplete absorption.
Pravastatin is rapidly absorbed from the upper part of the small intestine via proton-coupled carrier-mediated transport to be later taken up in the livery by the sodium-independent bile acid transporter. The reported time to reach the peak serum concentration in the range of 30-55 mcg/L is of 1-1.5 hours with an AUC ranging from 60-90 mcg.h/L.
Toxicity
The reported oral LD50 of pravastatin in mice is of 8939 mg/kg. There haven't been significant overdosage reports however, in the case of overdosage, symptomatic treatment is recommended along with laboratory monitoring and supportive measures.
In carcinogenic studies, high dose administration of pravastatin has been reported to increase the incidence of hepatocellular carcinomas in males and lung carcinomas in females. There is no evidence relating the administration of pravastatin with mutagenicity in different assays not to produce effects in fertility or reproductive potential.
Description
Pravastatin is the third HMG-CoA reductase inhibitor introduced for the treatment of atherosclerosis. Compared with lovastatin and simvastatin launched earlier, pravastatin is equipotent as an HMG-CoA reductase inhibitor in vitro, yet it is reported to be more tissue-selective.
Chemical properties
Off-white Cryst
Originator
Sankyo (Japan)
The Uses of Pravastatin
antiglaucoma,
The Uses of Pravastatin
anti-hyperlipoproteinemic, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor
Background
Pravastatin is the 6-alpha-hydroxy acid form of mevastatin. Pravastatin was firstly approved in 1991 becoming the second available statin in the United States. It was the first statin administered as the active form and not as a prodrug. This drug was developed by Sankyo Co. Ltd.; however, the first approved pravastatin product was developed by Bristol Myers Squibb and FDA approved in 1991.
Pravastatin is made through a fermentation process in which mevastatin is first obtained. The manufacturing process is followed by the hydrolysis of the lactone group and the biological hydroxylation with Streptomyces carbophilus to introduce the allylic 6-alcohol group.
Indications
Pravastatin is indicated for primary prevention of coronary events hypercholesterolemic patients without clinical evidence of coronary heart disease. Its use includes the reduction of risk on myocardial infarction, undergoing myocardial revascularization procedures and cardiovascular mortality.
As well, pravastatin can be used as a secondary prevention agent for cardiovascular events in patients with clinically evident coronary heart disease. This indication includes the reduction of risk of total mortality by reducing coronary death, myocardial infarction, undergoing myocardial revascularization procedures, stroke, and stroke/transient ischemic attack as well as to slow the progression of coronary atherosclerosis.
The term cardiovascular events correspond to all the incidents that can produce damage to the heart muscle including the interruption of blood flow.
As adjunctive therapy to diet, pravastatin is used in:
In patients that do not respond adequately to diet, pravastatin is used to treat patients with primary dysbetalipoproteinemia (type III hyperlipidemia).
Dyslipidemia is defined as an elevation of plasma cholesterol, triglycerides or both as well as to the presence of low levels of high-density lipoprotein. This condition represents an increased risk for the development of atherosclerosis.
What are the applications of Application
Pravastatin is a competitive HMGCR inhibitor
Definition
ChEBI: Pravastatin is a carboxylic ester resulting from the formal condensation of (S)-2-methylbutyric acid with the hydroxy group adjacent to the ring junction of (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6,8-dihydroxy-2-methyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid. Derived from microbial transformation of mevastatin, pravastatin is a reversible inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA). The sodium salt is used for lowering cholesterol and preventing cardiovascular disease. It is one of the lower potency statins, but has the advantage of fewer side effects compared with lovastatin and simvastatin. It has a role as a metabolite, an anticholesteremic drug, a xenobiotic and an environmental contaminant. It is a 3-hydroxy carboxylic acid, a hydroxy monocarboxylic acid, a carboxylic ester, a secondary alcohol, a carbobicyclic compound and a statin (semi-synthetic). It is functionally related to a (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6,8-dihydroxy-2-methyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid and a (S)-2-methylbutyric acid. It is a conjugate acid of a pravastatin(1-).
Manufacturing Process
Pravastatin was isolated as products of enzymatic hydroxylation by some kinds of microorganisms of [1S-[1-α(R*),7β,8β(2S*,4S*)8αβ]]-2methylbutanoic acid 1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-(tetrahydro-4hydroxy-6-oxo-2))-pyran-2-yl)ethyl]-1-naphthalenic lactone (campactin) or their carboxylic acid or their salts (products of animal metabolism of microorganisms from the genera Nocardia, Streptomyces et cetera).
Pravastatin may be preparated by using the microorganisms of genera Nocardia (method 1) and Mortierella (method 2).
Method 1
Cultivation of Nocardia autotrophica subsp. amethystineCells of Nocardia autotrophica subsp. amethystina FERM P-6183 was inoculated from a slant culture by means of a platinum loop into each of twenty 500 ml Erlenmeyer flasks, each containing 100 ml of a culture medium having the following composition: glucose - 1.0%, peptone - 0.2%, meat extract - 0.1%, yeast extract - 0.1%, corn steep liquor 0.3%, tap water balance..
Shaking was then carried out at 26°C and 220 r.p.m. for 2 days, at which time sodium 2-methyl-8-(2-methyl-1-oxobutoxy)-β,δ-dihydroxy(1S-(1-α(βS*,δ-S*),2-α,6-α,8-β(R*),8a-α))-1-1,2,6,7,8,8ahexahydronaphthaleneheptanoate was added to a final concentration of 0.05% w/v. Incubation was continued at 26°C and 220 r.p.m. for a further 5 days.
Preparation of pravastatin
After completion of the cultivation, the reaction mixture was filtered and the pH of the filtrate was adjusted to a value of 3 by the addition of trifluoroacetic acid. The acidified filtrate was then extracted three times, each with 1 liter of ethyl acetate, to give extracts containing a mixture (6-α and 6-β) of (1S-(1α,β- S*,δ-S*),2-α,8-βR*),8a-α))-1-naphthaleneheptanoic acid 1,2,6,7,8,8ahexahydro-2-methyl-8-(2-methyl-1-oxobutoxy)-β,δ,6-trihydroxy.
This extract was then immediately transferred into a 5% w/v aqueous solution of sodium hydrogen carbonate, and the pH of the mixture was adjusted to a value of 7.0 by the addition of 2 N hydrochloric acid. The mixture was then adsorbed on a Diaion HP-20 column. The column was washed with water and then eluted with 50% v/v aqueous acetone to give a fraction containing (1S(1-α,β- S*,δ-S*),2-α,6-α,8-βR*),8a-α))-1-naphthaleneheptanoic acid 1,2,6,7,8,8a-hexahydro-2-methyl-8-(2-methyl-1-oxobutoxy)-β,δ,6-trihydroxy-, monosodium salt (pravastatin). This was freeze-dried, to give 200 mg of pravastatine.
Method 2
Cultivation of Mortierella maculata nov. spec. E-97 [NCAIM(P)F 001266]
A spore suspension was prepared with 5 ml of a 0.9% sodium chloride solution obtained from a 7-10 day old, malt extract-yeast extract agar slant culture of Mortierella maculata nov. spec. E-97 [NCAIM(P)F 001266] strain able to 6-β-hydroxylate compactin and the suspension was used to inoculate 100 ml inoculum medium PI (glucose-50 g, soybean meal-20 g, in 1000 ml tap water) sterilized in a 500 ml Erlenmeyer flask.
5 liters working volume a bioconversion culture medium is prepared (glucose20 g, glycerine-20 g, soybean meal-20 g, peptone-5 g, potassium dihydrogen phosphate-0.5 g, polypropylene glycol 2000-1 g, in 1000 ml tap water); the components of the culture medium are added corresponding to 5 liters. Then it was sterilized for 45 min at 121°C and seeded with 500 ml of the inoculum culture.
Before sterilization the pH of the medium was adjusted to 7.0 value.
The fermentation was carried out at 28°C, with a stirring rate of 400 rpm andwith an aeration rate from bottom direction 60 liters/hour for 4 days. At the 2nd day after the transfer the culture started to foam heavily, which can be decreased by the addition of further polypropylene glycol 2000. The pH reached 6.3-7.5 by the 4th day. The feeding of the sodium 2-methyl-8-(2methyl-1-oxobutoxy)-β,δ-dihydroxy(1S-(1-α(β- S*,δ-S*),2-α,6-α,8-β(R*),8aα))-1-1,2,6,7,8,8a-hexahydronaphthaleneheptanoate substrate is allowed to be started if the pH of the broth is above 6.3.
Preparation of pravastatinAt the 4th day of the fermentation 2.5 g compactin substrate is added in sterile filtered aqueous solution. Calculated for the volume of the broth 0.51.0% glucose was added into the culture depending on the pH in the form of 50% solution sterilized at 121°C for 25 min in parallel with the substrate feeding. After 24 hours the compactin substrate is consumed from the culture (is detected by HPLC) and was converted to pravastatin. By lyophilization of the aqueous residue 1.3 g pravastatin was obtained. The chromatographically pure product was crystallized from a mixture of ethanol and ethyl acetate. Melting point: 170-173°C (decomp.).
brand name
Pravachol (Bristol-Myers Squibb);Mevalotin.
Therapeutic Function
Antihyperlipidemic
General Description
Pravastatin, sodium 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-1-naphthaleneheptanoate (Pravachol), is the most rapid actingof the three HMG-CoA reductase inhibitor drugs, reachinga peak concentration in about 1 hour. The sodium salt of theβ-hydroxy acid is more hydrophilic than the lactone forms ofthe other two agents, which may explain this property. In addition,the open form of the lactone ring contributes to a morehydrophilic agent, which, in turn, results in less CNS penetration.This explains, in part, why pravastatin has fewer CNSside effects than the more lipophilic lactone ester of this classof agents. Absorption of pravastatin following oral administrationcan be inhibited by resins such as cholestyramine becauseof the presence of the carboxylic acid function on thedrug. The lactone forms of lovastatin and simvastatin are lessaffected by cholestyramine.
Pharmacokinetics
The action of pravastatin on the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase produces an increase in the expression of hepatic LDL receptors which in order decreases the plasma levels of LDL cholesterol.
The effect of pravastatin has been shown to significantly reduce the circulating total cholesterol, LDL cholesterol, and apolipoprotein B. As well, it modestly reduces very low-density-lipoproteins (VLDL) cholesterol and triglycerides while increasing the level of high-density lipoprotein (HDL) cholesterol and apolipoprotein A.
In clinical trials with patients with a history of myocardial infarction or angina with high total cholesterol, pravastatin decreased the level of total cholesterol by 18%, decreased of LDL by 27%, decreased of triglycerides by 6% and increased of high-density lipoprotein (HDL) by 4%. As well, there was reported a decrease in risk of death due to coronary disease of 24%.
When coadministered with cholestyramine, pravastatin can reduce by 50% the levels of LDL and slow the progression of atherosclerosis and the risk of myocardial infarction and death.
Metabolism
After initial administration, pravastatin undergoes extensive first-pass extraction in the liver. However, pravastatin's metabolism is not related to the activity of the cytochrome P-450 isoenzymes and its processing is performed in a minor extent in the liver. Therefore, this drug is highly exposed to peripheral tissues.
The metabolism of pravastatin is ruled mainly by the presence of glucuronidation reactions with very minimal intervention of CYP3A enzymes. After metabolism, pravastatin does not produce active metabolites. This metabolism is mainly done in the stomach followed by a minor portion of renal and hepatic processing.
The major metabolite formed as part of pravastatin metabolism is the 3-alpha-hydroxy isomer. The activity of this metabolite is very clinically negligible.
Properties of Pravastatin
| Melting point: | 171.2-173 °C |
| Boiling point: | 634.5±55.0 °C(Predicted) |
| Density | 1.21±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: 19 mg/mL |
| form | powder |
| pka | 4.31±0.10(Predicted) |
| color | white |
| CAS DataBase Reference | 81093-37-0(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Naphthaleneheptanoic acid, 1,2,6,7,8,8a-hexahydro-.beta.,.delta.,6-trihydroxy-2-methyl-8-[(2S)-2-methyl-1-oxobutoxy]-, (.beta.R,.delta,R,1S,2S,6S,8S,8aR)- (81093-37-0) |
Safety information for Pravastatin
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H360:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Pravastatin
Pravastatin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1