CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Boiling Point | >600 ºC at 760 mmHg |
| Melting Point | 171.2 - 173°C |
| Solubility | 10 mg/ml |
| LogP | 0.59 |
| Stability/Shelf Life | Stable under recommended storage conditions. /Pravastatin sodium salt hydrate/ |
| Dissociation Constants | 4.2 |
| Collision Cross Section | 206.51 Ų [M-H]- |
| Other Experimental Properties | MW: 446.52. Odorless, white to off-white, fine or crystalline powder. Hygroscopic. UV max (ethanol): 230, 237, 245 nm. Freely soluble in water, methanol; soluble in alcohol; slightly soluble in isopropanol; very slightly soluble in acetonitrile. Practically insoluble in acetone, ethyl acetate, chlroform, ether. /Pravastatin sodium/ |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H360:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 424.5 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 424.24610348 g/mol |
| Monoisotopic Mass | 424.24610348 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 656 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
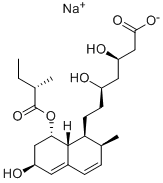
Pravastatin is a carboxylic ester resulting from the formal condensation of (S)-2-methylbutyric acid with the hydroxy group adjacent to the ring junction of (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6,8-dihydroxy-2-methyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid. Derived from microbial transformation of mevastatin, pravastatin is a reversible inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA). The sodium salt is used for lowering cholesterol and preventing cardiovascular disease. It is one of the lower potency statins, but has the advantage of fewer side effects compared with lovastatin and simvastatin. It has a role as a metabolite, an anticholesteremic drug, a xenobiotic and an environmental contaminant. It is a 3-hydroxy carboxylic acid, a hydroxy monocarboxylic acid, a carboxylic ester, a secondary alcohol, a carbobicyclic compound and a statin (semi-synthetic). It is functionally related to a (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6,8-dihydroxy-2-methyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid and a (S)-2-methylbutyric acid. It is a conjugate acid of a pravastatin(1-).