Pitavastatin
- CAS NO.:147511-69-1
- Empirical Formula: C25H24FNO4
- Molecular Weight: 421.46
- MDL number: MFCD04113054
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
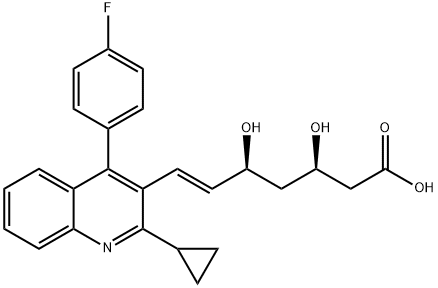
What is Pitavastatin?
Absorption
Pitavastatin peak plasma concentrations are achieved about 1 hour after oral administration. Both Cmax and AUC0-inf increased in an approximately dose-proportional manner for single pitavastatin doses from 1 mg to 24 mg once daily. The absolute bioavailability of pitavastatin oral solution is 51%. The Cmax and AUC of pitavastatin did not differ following evening or morning drug administration. In healthy volunteers receiving 4 mg pitavastatin, the percent change from baseline for LDL-C following evening dosing was slightly greater than that following morning dosing. Pitavastatin was absorbed in the small intestine but very little in the colon.
Administration of pitavastatin with a high fat meal (50% fat content) decreases pitavastatin Cmax by 43% but does not significantly reduce pitavastatin AUC.
Compared to other statins, pitavastatin has a relatively high bioavailability, which has been suggested to occur due to enterohepatic reabsorption in the intestine following intestinal absorption.
Genetic differences in the OATP1B1 (organic-anion-transporting polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact pitavastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) in the gene encoding OATP1B1 (SLCO1B1) demonstrated that pitavastatin AUC was increased 3.08-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. Other statin drugs impacted by this polymorphism include simvastatin, pitavastatin, atorvastatin, and rosuvastatin. Individuals with the 521CC genotype may be at increased risk of dose-related adverse effects including myopathy and rhabdomyolysis due to increased exposure to the drug.
Toxicity
Pitavastatin decreases synthesis of cholesterol and possibly other biologically active substances derived from cholesterol; therefore, pitavastatin may cause fetal harm when administered to pregnant patients based on the mechanism of action. In addition, treatment of hyperlipidemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients.
A Medicaid cohort linkage study of 1152 statin-exposed pregnant women compared to 886,996 controls did not find a significant teratogenic effect from maternal use of statins in the first trimester of pregnancy, after adjusting for potential confounders – including maternal age, diabetes mellitus, hypertension, obesity, and alcohol and tobacco use – using propensity score-based methods. The relative risk of congenital malformations between the group with statin use and the group with no statin use in the first trimester was 1.07 (95% confidence interval 0.85 to 1.37) after controlling for confounders, particularly pre-existing diabetes mellitus. There were also no statistically significant increases in any of the organ-specific malformations assessed after accounting for confounders. In the majority of pregnancies, statin treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. Study limitations include reliance on physician coding to define the presence of a malformation, lack of control for certain confounders such as body mass index, use of prescription dispensing as verification for the use of a statin, and lack of information on non-live births.
No specific treatment for pitavastatin overdose is known. Contact Poison Control (1-800-222-1222) for latest recommendations. Hemodialysis is unlikely to be of benefit due to high protein binding ratio of pitavastatin.
In a 92-week carcinogenicity study in mice given pitavastatin, at the maximum tolerated dose of 75 mg/kg/day with systemic maximum exposures (AUC) 26 times the clinical maximum exposure
at 4 mg daily, there was an absence of drug-related tumors.
In a 92-week carcinogenicity study in rats given pitavastatin at 1, 5, 25 mg/kg/day by oral gavage there was a significant increase in the incidence of thyroid follicular cell tumors at 25 mg/kg/day, which represents 295 times human systemic exposures based on AUC at the 4 mg daily maximum human dose. In a 26-week transgenic mouse (Tg rasH2) carcinogenicity study where animals were given pitavastatin at 30, 75, and 150 mg/kg/day by oral gavage, no clinically significant tumors were observed.
Pitavastatin was not mutagenic in the Ames test with Salmonella typhimurium and Escherichia coli with and without metabolic activation, the micronucleus test following a single administration in mice and multiple administrations in rats, the unscheduled DNA synthesis test in rats, and a Comet assay in mice. In the chromosomal aberration test, clastogenicity was observed at the highest doses tested, which also elicited high levels of cytotoxicity.
Pitavastatin had no adverse effects on male and female rat fertility at oral doses of 10 and 30 mg/kg/day, respectively, at systemic exposures 56- and 354-times clinical exposure at 4 mg daily based on AUC.
Pitavastatin treatment in rabbits resulted in mortality in males and females given 1 mg/kg/day (30-times clinical systemic exposure at 4 mg daily based on AUC) and higher during a fertility study. Although the cause of death was not determined, rabbits had gross signs of renal toxicity (kidneys whitened) indicative of possible ischemia. Lower doses (15-times human systemic exposure) did not show significant toxicity in adult males and females. However, decreased implantations, increased resorptions, and decreased viability of fetuses were observed.
The Uses of Pitavastatin
HMGA reductase inhibitor
Indications
Pitavastatin is indicated for the treatment of adult patients with primary hyperlipidemia or mixed dyslipidemia to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C). It is also indicated for the treatment of pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH) to reduce elevated TC, LDL-C, and Apo B.
Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Background
Pitavastatin, also known as the brand name product Livalo, is a lipid-lowering drug belonging to the statin class of medications. By inhibiting the endogenous production of cholesterol within the liver, statins lower abnormal cholesterol and lipid levels and ultimately reduce the risk of cardiovascular disease. More specifically, statin medications competitively inhibit the enzyme hydroxymethylglutaryl-coenzyme A (HMG-CoA) Reductase, which catalyzes the conversion of HMG-CoA to mevalonic acid. This is the third step in a sequence of metabolic reactions involved in the production of several compounds involved in lipid metabolism and transport including cholesterol, low-density lipoprotein (LDL) (sometimes referred to as "bad cholesterol"), and very low-density lipoprotein (VLDL). Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD, such as those with Type 2 Diabetes. The clear evidence of the benefit of statin use coupled with very minimal side effects or long term effects has resulted in this class becoming one of the most widely prescribed medications in North America.
Pitavastatin and other drugs from the statin class of medications including atorvastatin, pravastatin, rosuvastatin, fluvastatin, and lovastatin are considered first-line options for the treatment of dyslipidemia. Increasing use of the statin class of drugs is largely due to the fact that cardiovascular disease (CVD), which includes heart attack, atherosclerosis, angina, peripheral artery disease, and stroke, has become a leading cause of death in high-income countries and a major cause of morbidity around the world. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks.
While all statin medications are considered equally effective from a clinical standpoint, rosuvastatin is considered the most potent; doses of 10 to 40mg rosuvastatin per day were found in clinical studies to result in a 45.8% to 54.6% decrease in LDL cholesterol levels. Study data has confirmed that pitavastatin's potency in lowering LDL-C is comparable to that of other statins but also has increased efficacy in increasing HDL-C (also known as "good cholesterol"). Despite these differences in potency, several trials have demonstrated only minimal differences in terms of clinical outcomes between statins.
Definition
ChEBI: A hydroxy monocarboxylic acid anion that is the conjugate base of pitavastatin, obtained by deprotonation of the carboxy group.
brand name
[Name previously used: Itavastatin].
Biological Activity
pitavastatin (nk-104) is a potent hmg-coa reductase inhibitor, pitavastatin inhibited cholesterol synthesis from acetic acid with an ic50 of 5.8 nm in a human liver cancer cell line (hepg2).
Pharmacokinetics
Pitavastatin is an oral antilipemic agent which inhibits HMG-CoA reductase. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, rosuvastatin reduces the risk of cardiovascular morbidity and mortality.
Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks.
Skeletal Muscle Effects
Pitavastatin may cause myopathy (muscle pain, tenderness, or weakness with creatine kinase (CK) above ten times the upper limit of normal) and rhabdomyolysis (with or without acute renal failure secondary to myoglobinuria). Rare fatalities have occurred as a result of rhabdomyolysis with statin use, including pitavastatin. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. As dosages of pitavastatin greater than 4mg per day were associated with an increased risk of severe myopathy, the product monograph recommends a maximum daily dose of 4mg once daily.
The risk of myopathy during treatment with pitavstatin may be increased with concurrent administration of interacting drugs such as fenofibrate, niacin, gemfibrozil, and cyclosporine. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with colchicine, and caution should therefore be exercised when prescribing these two medications together.
Real-world data from observational studies has suggested that 10-15% of people taking statins may experience muscle aches at some point during treatment.
Hepatic Dysfunction
Increases in serum transaminases have been reported with pitavastatin. In most cases, the elevations were transient and either resolved or improved on continued therapy or after a brief interruption in therapy. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including pitavastatin.
Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury.
Increases in HbA1c and Fasting Serum Glucose Levels
Increases in HbA1c and fasting serum glucose levels have been reported with statins, including pitavastatin. Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices.
An in vitro study found that atorvastatin, pravastatin, rosuvastatin, and pitavastatin exhibited a dose-dependent cytotoxic effect on human pancreas islet β cells, with reductions in cell viability of 32, 41, 34 and 29%, respectively, versus control. Moreover, insulin secretion rates were decreased by 34, 30, 27 and 19%, respectively, relative to control.
Enzyme inhibitor
This HMG-CoA reductase inhibitor (FW = 421.46 g/mol; CAS 147511-69- 1; IUPAC Name: (3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin- 3-yl]-3,5-dihydroxyhept-6-enoic acid), also known as itavastatin, itabavastin, nisvastatin, NK-104, NKS-104, and the trade name Livalo?, is indicated for ameliorating hypercholesterolemia and preventing cardiovascular disease. NK-104 potency is dose-dependent and is roughly equivalent to that of atorvastatin. It is well-tolerated in the treatment of patients with hypercholesterolemia. Pitavastatin uptake is carrier-mediated. Target(s): Reduces inflammatory cytokine production from human bronchial epithelial cells; Decreases microtubule tau protein levels via the inactivation of Rho/ROCK; Inhibits hepatic steatosis and fibrosis in non-alcoholic steatohepatitis model; Suppresses therosclerosis induced by chronic inhibition of the synthesis of nitric oxide in moderately hypercholesterolemic rabbits; Decreases the expression of endothelial lipase both in vitro and in vivo; Inhibits NFkB pathway in brain; Inactivates NFkB and decreases IL-6 production through Rho kinase pathway in MCF-7 human breast cancer cells; Reduces C-reactive-protein-induced interleukin-8 production in human aortic endothelial cells; Inhibits lysophosphatidic acid-induced proliferation and monocyte chemoattractant protein-1 expression in aortic smooth muscle cells by suppressing Rac-1-mediated reactive oxygen species generation; Inhibits upregulation of intermediate conductance calcium-activated potassium channels and coronary arteriolar remodeling induced by long-term blockade of nitric oxide synthesis; Inhibits migration and proliferation of rat vascular smooth muscle cells.
Metabolism
The principal route of pitavastatin metabolism is glucuronidation via liver uridine 5'-diphosphate glucuronosyltransferase (UGT) with subsequent formation of pitavastatin lactone. There is only minimal metabolism by the cytochrome P450 system. Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite in human plasma is the lactone, which is formed via an ester-type pitavastatin glucuronide conjugate by UGTs (UGT1A3 and UGT2B7).
Properties of Pitavastatin
| Melting point: | 182 - 185°C |
| Boiling point: | 692.0±55.0 °C(Predicted) |
| Density | 1.352±0.06 g/cm3(Predicted) |
| storage temp. | -20°C, Hygroscopic |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.24±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 147511-69-1(CAS DataBase Reference) |
| EPA Substance Registry System | 6-Heptenoic acid, 7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-, (3R,5S,6E)- (147511-69-1) |
Safety information for Pitavastatin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pitavastatin
Pitavastatin manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
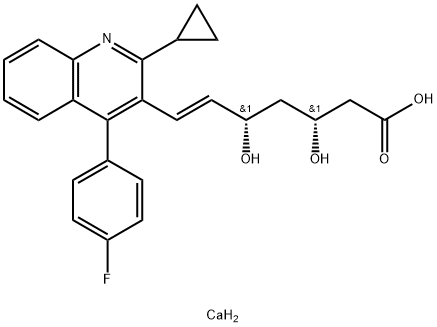

You may like
-
 147511-69-1 Pitavastatin 98%View Details
147511-69-1 Pitavastatin 98%View Details
147511-69-1 -
 Pitavastatin 98%View Details
Pitavastatin 98%View Details
147511-69-1 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 201530-41-8 Deferasirox 98%View Details
201530-41-8 Deferasirox 98%View Details
201530-41-8 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
