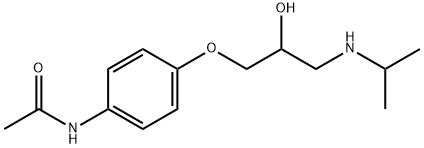
Practolol
- CAS NO.:6673-35-4
- Empirical Formula: C14H22N2O3
- Molecular Weight: 266.34
- MDL number: MFCD00864573
- EINECS: 229-712-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-09-20 16:43:00
What is Practolol?
Toxicity
Symptoms of overdose include abdominal irritation, central nervous system depression, coma, extremely slow heartbeat, heart failure, lethargy, low blood pressure, and wheezing.
Chemical properties
N-[4-[2-HYDROXY-3-[(1-METHYLETHYL)AMINO]PROPOXY]PHENYL]ACETAMIDE is White Solid
Originator
Eraldin, I.C.I. ,UK,1970
The Uses of Practolol
Practolol is a selective adrenergic β-receptor blocking drug with antihyperstensive activity. Practolol has been used in the emergency treatment of cardiac arrhythmias.
The Uses of Practolol
N-[4-[2-HYDROXY-3-[(1-METHYLETHYL)AMINO]PROPOXY]PHENYL]ACETAMIDE is a selective adrenergic β-receptor blocking drug with antihyperstensive activity. N-[4-[2-HYDROXY-3-[(1-METHYLETHYL)AMINO]PROPOXY]PHENYL]ACETAMIDE has been used in the emergency treatment of cardiac arrhythmias.
Indications
Used in the emergency treatment of cardiac arrhythmias.
Background
A beta-adrenergic antagonist that has been used in the emergency treatment of cardiac arrhythmias.
Definition
ChEBI: N-(4-Hydroxyphenyl)acetamide in which the hydrogen of the phenolic hydroxy group is substituted by a 3-(isopropylaminoamino)-2-hydroxypropyl group. A selective beta blocker, it has been used in the emergency treatment of cardiac arrhythm as.
Manufacturing Process
The 1-(4-acetamidophenoxy)-2,3-epoxypropane used as starting material may be obtained as follows. To a solution of 4.5 parts of 4-acetamidophenol and 1.5 parts of sodium hydroxide in 50 parts of water at 15°C, there is added 3.5 parts of epichlorohydrin. The mixture is stirred for 16 hours at ambient temperature, filtered and the solid residue is washed with water. There is thus obtained 1-(4-acetamidophenoxy)-2,3-epoxypropane, MP 110°C.
A mixture of 2 parts of 1-(4-acetamidophenoxy)-2,3-epoxypropane and 10 parts of isopropylamine is stirred at ambient temperature for 16 hours. The resulting solution is evaporated to dryness under reduced pressure and the residue is crystallized from butyl acetate. There is thus obtained 1-(4acetamidophenoxy)-3-isopropylamino-2-propanol, MP 134° to 136°C.
brand name
A 25;Cardiol;Cordialina;Eraldina;Pralon;Teranol.
Therapeutic Function
Antiarrhythmic
World Health Organization (WHO)
Practolol, a beta-adrenoreceptor antagonist, was introduced in 1970 for the treatment of angina and cardiac dysrhythmias. By 1974 long-term use had been associated with serious delayed idiosyncratic reactions (oculomucocutaneous syndrome) and this led to the withdrawal of oral preparations by the major manufacturer on a worldwide basis. There is no evidence to suggest that other beta-adrenoreceptor antagonist are associated with this risk. Intravenous preparations of practolol remain available in many countries for the emergency treatment of selected cardiac dysrhythmias.
Biological Activity
Practolol is a potent and selective β1 adrenergic receptor antagonist. It can be used in the study of cardiac arrhythmias.
Pharmacokinetics
Practolol is a beta-adrenergic receptor antagonist that has been used in the emergency treatment of cardiac arrhythmias. Beta blockers inhibit normal epinephrine-mediated sympathetic actions, but have minimal effect on resting subjects. That is, they reduce the effect of excitement/physical exertion on heart rate and force of contraction and dilation of blood vessels.
in vitro
Practolol (10 μM; 30 min) preferentially antagonizes the relaxant effects produced by R0363 in spontaneously contracted tracheal preparations from the guinea-pig.Practolol (10 nM-100 μM) blocks the progesterone production induced by (-)epinephrine in a dose-dependent manner in granulosa cells.
in vivo
Practolol (0.5 mg/kg; i.v.) decreases heart rate, left ventricular dP/dt max , myocardial blood flow and cardiac output in intact close-chest dogs.Practolol (0.5 mg/kg; i.v.) decreases normal myocardial blood flow but flow in the ischaemic area remains unchanged after coronary artery ligation.
Metabolism
Not Available
Properties of Practolol
| Melting point: | 134-136° (BuOAc) |
| Boiling point: | 409.54°C (rough estimate) |
| Density | 1.0807 (rough estimate) |
| refractive index | 1.5110 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | pKa 9.5 (Uncertain) |
| form | Solid |
| color | White to Off-White |
Safety information for Practolol
Computed Descriptors for Practolol
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
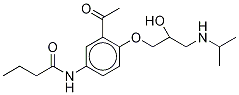

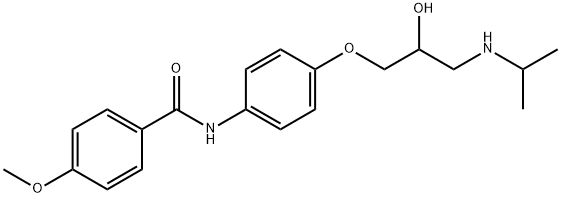
![N-[3-ACETYL-4-[2-HYDROXY-3-[(1-METHYLETHYL)AMINO]PROPOXY]PHENYL]BUTANAMIDE](https://img.chemicalbook.in/CAS/GIF/28197-63-9.gif)
You may like
-
 rac Practolol 95% (HPLC) CAS 6673-35-4View Details
rac Practolol 95% (HPLC) CAS 6673-35-4View Details
6673-35-4 -
 Practolol CAS 6673-35-4View Details
Practolol CAS 6673-35-4View Details
6673-35-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8