Acebutolol hydrochloride
Synonym(s):N-(3-Acetyl-4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl)butanamide;Acebutolol hydrochloride
- CAS NO.:34381-68-5
- Empirical Formula: C18H29ClN2O4
- Molecular Weight: 372.89
- MDL number: MFCD00078860
- EINECS: 251-980-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
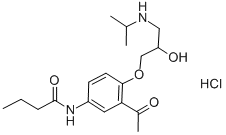
What is Acebutolol hydrochloride?
Chemical properties
Off-White Powder
Originator
Sectral ,May and Baker ,UK ,1975
The Uses of Acebutolol hydrochloride
Cardioselective ?adrenergic blocker. Antihypertensive; antianginal; antiarrhythmic (class II)
The Uses of Acebutolol hydrochloride
Cardioselective β-adrenergic blocker. Antihypertensive; antianginal; antiarrhythmic (class II).
The Uses of Acebutolol hydrochloride
5-alpha-reductase inhibitor
What are the applications of Application
Acebutolol Hydrochloride is a β-AR antagonist
Definition
ChEBI: The hydrochloride salt of acebutolol, prepared using equimolar amounts of acebutolol and hydrogen chloride.
Manufacturing Process
Crude 5'-butyramido-2'-(2,3-epoxypropoxy)acetophenone (16 g),
isopropylamine (20 g) and ethanol (100 ml) were heated together under
reflux for 4 hours. The reaction mixture was concentrated under reduced
pressure and the residual oil was dissolved in N hydrochloric acid. The acid
solution was extracted with ethyl acetate, the ethyl acetate layers being
discarded. The acidic solution was brought to pH 11 with 2 N aqueous sodium
hydroxide solution and then extracted with chloroform. The dried chloroform
extracts were concentrated under reduced pressure to give an oil which was
crystallized from a mixture of ethanol and diethyl ether to give 5'-butyramido-
2'-(2-hydroxy-3-isopropylaminopropoxy)acetophenone (3 g), MP 119-123°C.
Crude 5'-butyramido-2'-(2,3-epoxypropoxy)acetophenone used as starting
material was prepared as follows: p-butyramidophenol (58 g; prepared
according to Fierz-David and Kuster, Helv. Chim. Acta 1939,2282), acetyl
chloride (25.4 g) and benzene (500 ml) were heated together under reflux
until a solution formed (12 hours). This solution was cooled and treated with
water. The benzene layer was separated and the aqueous layer was again
extracted with benzene.
The combined benzene extracts were dried and evaporated to dryness under
reduced pressure to give p-butyramidophenyl acetate (38 g) as an off-white
solid, MP 102-103°C. A mixture of p-butyramidophenyl acetate (38 g),
aluminum chloride (80 g) and 1,1,2,2-tetrachloroethane (250 ml) was heated
at 140°C for 3 hours. The reaction mixture was cooled and treated with iced
water. The tetrachloroethane layer was separated and the aqueous layer was
extracted with chloroform. The combined organic layers were extracted with 2
N aqueous sodium hydroxide and the alkaline solution was acidified to pH 5
with concentrated hydrochloric acid. The acidified solution was extracted with
chloroform and the chloroform extract was dried and concentrated under
reduced pressure to give 5'-butyramido-2'-hydroxyacetophenone (15.6 g), MP
114-117°C. A solution of 5'-butyramido-2'-hydroxyacetophenone (15.6 g) in ethanol (100 ml) was added to an ethanolic solution of sodium ethoxide which
was prepared from sodium (1.62 g) and ethanol (100 ml). The resulting
solution was evaporated to dryness under reduced pressure and
dimethylformamide (100 ml) was added to the solid residue. Approximately
10 ml of dimethylformamide was removed by distillation under reduced
pressure. Epichlorohydrin (25 ml) was added and the solution was heated at
100°C for 4 hours. The solution was concentrated under reduced pressure to
give a residual oil which was treated with water to give a solid. The solid was
dissolved in ethanol and the resulting solution was treated with charcoal,
filtered and concentrated under reduced pressure to give crude 5'-butyramido-
2'-(2,3-epoxypropoxy)acetophenone (16 g), MP 110-116°C.
The crude compound may be purified by recrystallization from ethyl acetate,
after treatment with decolorizing charcoal, to give pure 5'-butyramido-2'-(2,3-
epoxypropoxy)acetophenone, MP 136-138°C.
brand name
Sectral (Dr. Reddy’s).
Therapeutic Function
beta-Adrenergic blocker
Safety Profile
Poison by ingestion, subcutaneous,intravenous, and intraperitoneal routes. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits toxic fumes of NOx andHCl.
Properties of Acebutolol hydrochloride
| Melting point: | 141-1430C |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water and in ethanol (96 per cent), very slightly soluble in acetone and in methylene chloride. |
| form | neat |
| form | Solid |
| color | White to Pale Yellow |
| CAS DataBase Reference | 34381-68-5(CAS DataBase Reference) |
Safety information for Acebutolol hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Acebutolol hydrochloride
| InChIKey | KTUFKADDDORSSI-UHFFFAOYSA-N |
Acebutolol hydrochloride manufacturer
Nuray Chemicals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Acebutolol hydrochloride 98%View Details
Acebutolol hydrochloride 98%View Details -
 34381-68-5 98%View Details
34381-68-5 98%View Details
34381-68-5 -
 Acebutolol HCl 97% CAS 34381-68-5View Details
Acebutolol HCl 97% CAS 34381-68-5View Details
34381-68-5 -
 Acebutolol hydrochloride 97% (HPLC) CAS 34381-68-5View Details
Acebutolol hydrochloride 97% (HPLC) CAS 34381-68-5View Details
34381-68-5 -
 Acebutolol hydrochloride CAS 34381-68-5View Details
Acebutolol hydrochloride CAS 34381-68-5View Details
34381-68-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1