Potassium trifluoromethanesulfonate
Synonym(s):Potassium triflate;Trifluoromethanesulfonic acid potassium salt
- CAS NO.:2926-27-4
- Empirical Formula: CF3KO3S
- Molecular Weight: 188.17
- MDL number: MFCD00042370
- EINECS: 207-009-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
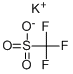
What is Potassium trifluoromethanesulfonate?
Chemical properties
white crystals or crystalline powder
The Uses of Potassium trifluoromethanesulfonate
Potassium trifluoromethanesulfonate is used in studies of mixed alkali effects and short range interactions in poly(ethylene oxide) electrolytes,1 and characteristics of the electrochemical behavior of glassy carbon in super-acid media.
The Uses of Potassium trifluoromethanesulfonate
Potassium trifluoromethanesulfonate is used as a reagent in the synthesis of guanine-quadruplex hybrid materials. It acts as a supporting electrolyte in the electrochemical study of evidence for gold anion in ethylenediamine. It is involved in the preparation of N-fluoro-2,4,6-trimethylpyridinium triflate by reacting with 2,4,6-trimethyl-pyridine. Further, it is used in the preparation of imidazolium salt, 3-methyl-1-(3R,3aR,6S,6aR)-[6-(benzyloxy)-hexahydrofuro[3,2-b]furan-3-yl]imidazolium trifluoromethanesulfonate.
General Description
Potassium trifluoromethanesulfonate (KOTf, potassium triflate) is the potassium salt of trifluoromethanesulphonic acid. It has been prepared by neutralizing a warm aqueous solution of trifluoromethanesulphonic acid with potassium carbonate. The structure of siloxane–poly(oxyethylene) hybrids doped with potassium triflate has been investigated.
structure and hydrogen bonding
According to the results of temperature dependent powder diffractometry potassium trifluoromethanesulfonate is dimorphic. The phase transition occurs between -63 degreesC and 45 degreesC. The low-temperature modification crystallizes monoclinic with a=10.300(3)Angstrom, b = 6.052(1) Angstrom, c = 14.710(4) Angstrom, beta =111.83(2)degrees (-120 degreesC) and the room-temperature modification with a=10.679(5) Angstrom, b=5.963(2)Angstrom, c=14.624(5)Angstrom, beta =111.57(3)degrees, Z=6, P2(1). According to single crystal structure determination, potassium trifluoromethanesulfonate consists of three different potassium-oxygen-coordination polyhedra, linked by sulfur atoms of the trifluoromethanesulfonate groups. This results in a channel structure with all lipophilic trifluoromethane groups pointing into these channels[1].
References
[1] Korus, G. , and M. Jansen . "Crystal structure, phase transition, and potassium ion conductivity of potassium trifluoromethanesulfonate." (2001).
Properties of Potassium trifluoromethanesulfonate
| Melting point: | 238.5°C |
| storage temp. | Inert atmosphere,Room Temperature |
| form | solid |
| color | White to Almost white |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 6: forms irreversible hydrate |
| BRN | 3727495 |
| InChI | InChI=1S/CHF3O3S.K/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1 |
| CAS DataBase Reference | 2926-27-4(CAS DataBase Reference) |
Safety information for Potassium trifluoromethanesulfonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium trifluoromethanesulfonate
| InChIKey | GLGXXYFYZWQGEL-UHFFFAOYSA-M |
| SMILES | C(F)(F)(F)S([O-])(=O)=O.[K+] |
Potassium trifluoromethanesulfonate manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
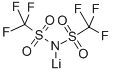
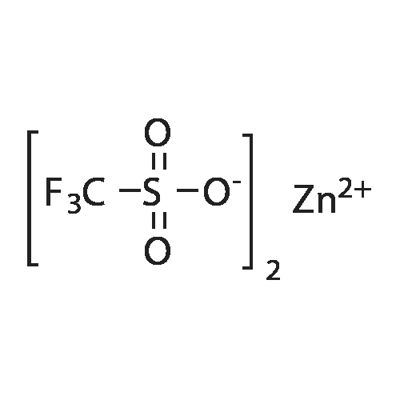
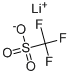
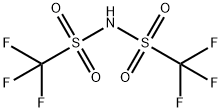

You may like
-
 Potassium trifluoromethanesulfonate 95% CAS 2926-27-4View Details
Potassium trifluoromethanesulfonate 95% CAS 2926-27-4View Details
2926-27-4 -
 Potassium Trifluoromethanesulfonate 98% (HPLC) CAS 2926-27-4View Details
Potassium Trifluoromethanesulfonate 98% (HPLC) CAS 2926-27-4View Details
2926-27-4 -
 Potassium trifluoromethanesulfonate, 98% CAS 2926-27-4View Details
Potassium trifluoromethanesulfonate, 98% CAS 2926-27-4View Details
2926-27-4 -
 Potassium Trifluoromethanesulfonate CAS 2926-27-4View Details
Potassium Trifluoromethanesulfonate CAS 2926-27-4View Details
2926-27-4 -
 Potassium trifluoromethanesulfonate CAS 2926-27-4View Details
Potassium trifluoromethanesulfonate CAS 2926-27-4View Details
2926-27-4 -
 2926-27-4 POTASSIUM TRIFLATE 99%View Details
2926-27-4 POTASSIUM TRIFLATE 99%View Details
2926-27-4 -
 Potassium trifluoromethanesulfonate CAS 2926-27-4View Details
Potassium trifluoromethanesulfonate CAS 2926-27-4View Details
2926-27-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
