Lithium bis(trifluoromethanesulphonyl)imide
Synonym(s):Bis(trifluoromethylsulfonyl)amine lithium salt;LiTFSI;Lithium bistrifluoromethanesulfonimidate
- CAS NO.:90076-65-6
- Empirical Formula: C2F6LiNO4S2
- Molecular Weight: 287.09
- MDL number: MFCD00210017
- EINECS: 415-300-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-14 13:52:13
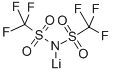
What is Lithium bis(trifluoromethanesulphonyl)imide?
Description
Lithium bis(trifluoromethylsulphonyl)imide (LiTFSI) is normally used as a p-dopant?to enhance the conductivity and hole mobility of the?Spiro-OMeTAD for perovskite solar cells. It is believed that The function of LiTFSI in PSCs is?similar to that in solid-state dye-sensitised solar cells [2].
Description
Lithium bis(trifluoromethane)sulfonimide (LiTFSI) is a hydrophilic organic salt that has many uses in electric and electronic systems. Its bis(trifluoromethane)sulfonimide anion, often referred to as bistriflimide, has the useful property of coordinating weakly with cations.
LiTFSI ‘s other important property is its extremely high solubility in water: 21 molal or ≈6 kg/L of solution. Because of its solubility, it has been explored as an electrolyte for lithium-ion batteries, solar cells, and similar applications since at least the late 1980s. LiTFSI is a safer material than the formerly used salt, lithium hexafluorophosphate (LiPF6).
In a 2021 Nature article, André D. Taylor at New York University (New York City) and Yale University (New Haven, CT) and 18 colleagues there and at other institutions in the United States and the Republic of Korea reported a refinement in the use of LiTFSI in solar cells.
Photovoltaic cells made of inexpensive light-absorbing perovskites that have high power-conversion efficiencies have been developed over the past several years. To improve the cells’ ability to transport electrical charges, they are doped with a combination of LiTFSI and a semiconductor called Spiro-OMeTAD1; but this process is extremely slow.
Taylor and his fellow researchers solved the problem by bubbling carbon dioxide into a solution of spiro-OMeTAD and LiTFSI while irradiating the mixture with ultraviolet light. They then cast a film made from the solution onto the perovskite light absorber. The process can be completed in ≈1 min, compared with the older, hours-long doping procedure.
The authors state, “The CO2-treated interlayer exhibits approximately 100 times higher conductivity than a pristine [untreated] film while realizing stable, high-efficiency solar cells without any post-treatments.” They also report that their method is useful for doping π-conjugated polymers.
1. 2,2′,7,7′-Tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9-spirobifluorene, CAS Reg. No. 207739-72-8.
Chemical properties
White hygroscopic powder
The Uses of Lithium bis(trifluoromethanesulphonyl)imide
It finds application in the preparation of rare-earth Lewis acid catalysts.
The Uses of Lithium bis(trifluoromethanesulphonyl)imide
Lithium bis(trifluoromethylsulfonyl)imide is used in the preparation of chiral imidazolium salt through an anion metathesis of the corresponding triflate organic electrolyte-based lithium batteries. It finds application in the preparation of rare-earth Lewis acid catalysts. It is also useful in primary and secondary lithium cells using organic liquid electrolytes and polymer batteries.
What are the applications of Application
Bis(trifluoromethane)sulfonimide lithium salt is an electrolyte
General Description
Bis(trifluoromethane)sulfonimide lithium salt is a synthetic reagent.
Flammability and Explosibility
Non flammable
Synthesis
5.91 g of anhydrous lithium fluoride and N-butyl-bistrifluoromethylsulfonimide 51.26 g. Add to 250 g of isopropyl acetate and reflux for 10 hours. It was filtered after being cooled to room temperature, and the filtrate was concentrated to dryness under reduced pressure.200 g of dichloromethane was added dropwise, and the mixture was stirred at 20 ° C for 2 hours and then filtered. Wash with dichloromethane. The filter cake was dried at 80 ° C to obtain 39.57 g of Lithium bis(trifluoromethanesulphonyl)imide. The yield is 90.7%.
Properties and Applications
Lithium bis(trifluoromethanesulphonyl)imide (LiTFSI) is a hydrophilic organic salt with many uses in electric and electronic systems. Its bis(trifluoromethane)sulfonimide anion, often called bistriflimide, is helpful in coordinating weakly with cations. LiTFSI's other important property is its extremely high solubility in water: 21 molal or ≈6 kg/L of solution. LiTFSI is safer than the formerly used salt, lithium hexafluorophosphate (LiPF6). To improve the cells' ability to transport electrical charges, researchers are doped with a combination of LiTFSI and a semiconductor called Spiro-OMeTAD1; however, this process is extremely slow. Taylor and his fellow researchers solved the problem by bubbling carbon dioxide into a solution of spiro-OMeTAD and LiTFSI while irradiating the mixture with ultraviolet light. They then cast a film from the solution onto the perovskite light absorber. The process can be completed in ≈1 minute, compared with the older, hours-long doping procedure.
Properties of Lithium bis(trifluoromethanesulphonyl)imide
| Melting point: | 234-238 °C(lit.) |
| Density | 1,334 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| Flash point: | >100°C (>212°F) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 10 mg/mL, clear, colorless |
| form | Hygroscopic Powder |
| appearance | hygroscopic white powder |
| color | White |
| Specific Gravity | 1.334 |
| Water Solubility | Soluble in water. |
| Sensitive | Moisture Sensitive |
| BRN | 6625414 |
| CAS DataBase Reference | 90076-65-6(CAS DataBase Reference) |
| EPA Substance Registry System | Methanesulfonamide, 1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]-, lithium salt (90076-65-6) |
| Boiling point: | 234-238?°C (lit.) |
Safety information for Lithium bis(trifluoromethanesulphonyl)imide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium bis(trifluoromethanesulphonyl)imide
| InChIKey | QSZMZKBZAYQGRS-UHFFFAOYSA-N |
Lithium bis(trifluoromethanesulphonyl)imide manufacturer
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 9-Anthracenemethanol, 97% Giemsa Stain, Modified Solution 1-Propynylmagnesium bromide 5-Bromo-1,2,3-trichlorobenzene, 95% 1-Bromo-4-chlorobenzene, 99% Benzocaine, 98% N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt Ethyl Methanesulfonate N Ethylmethylamine N N N'Trimethyl ethylenediamine (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Boc Leucine Boc-L-Tyr(tBu)-OH Fmoc-L-Glu-OtBu Sacubitril- Valsartan RamiprilRelated products of tetrahydrofuran


You may like
-
 Lithium triflimide, 99%+ CAS 90076-65-6View Details
Lithium triflimide, 99%+ CAS 90076-65-6View Details
90076-65-6 -
 Bis(trifluoromethane)sulphonimide lithium salt, 99.95% CAS 90076-65-6View Details
Bis(trifluoromethane)sulphonimide lithium salt, 99.95% CAS 90076-65-6View Details
90076-65-6 -
 Lithium bis(trifluoromethanesulfonyl)imide 95% CAS 90076-65-6View Details
Lithium bis(trifluoromethanesulfonyl)imide 95% CAS 90076-65-6View Details
90076-65-6 -
 Lithium bis(trifluoromethanesulfonyl)imide, anhydrous, 99.99% CAS 90076-65-6View Details
Lithium bis(trifluoromethanesulfonyl)imide, anhydrous, 99.99% CAS 90076-65-6View Details
90076-65-6 -
 Lithium Bis(trifluoromethanesulfonyl)imide CAS 90076-65-6View Details
Lithium Bis(trifluoromethanesulfonyl)imide CAS 90076-65-6View Details
90076-65-6 -
 Bis(trifluoromethane)sulfonimide lithium salt CAS 90076-65-6View Details
Bis(trifluoromethane)sulfonimide lithium salt CAS 90076-65-6View Details
90076-65-6 -
 Bis(trifluoromethane)sulfonimide lithium salt CAS 90076-65-6View Details
Bis(trifluoromethane)sulfonimide lithium salt CAS 90076-65-6View Details
90076-65-6 -
 Lithium bis(trifluoromethanesulfonyl)imide CAS 90076-65-6View Details
Lithium bis(trifluoromethanesulfonyl)imide CAS 90076-65-6View Details
90076-65-6
