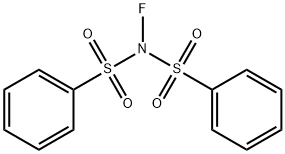
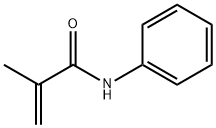
N-Phenyl-bis(trifluoromethanesulfonimide)
Synonym(s):N,N-Bis(trifluoromethylsulfonyl)aniline;N-Phenyl-trifluoromethanesulfonimide;1,1,1-Trifluoro-N-phenyl-N-[(trifluoromethyl)sulfonyl]methanesulfonamide;NSC 240874;Phenyl triflimide
- CAS NO.:37595-74-7
- Empirical Formula: C8H5F6NO4S2
- Molecular Weight: 357.25
- MDL number: MFCD00000404
- EINECS: 609-445-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-29 22:33:22
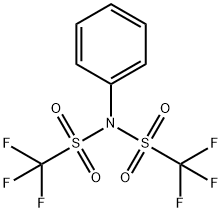
What is N-Phenyl-bis(trifluoromethanesulfonimide)?
Description
N-Phenylbis(trifluoromethanesulfonimide) acts as a mild triflating reagent as well as a transparent strong electron-withdrawing p-type dopant in carbon nanotubes. It is also employed as a reactant for the preparation of amphoteric alfa-boryl aldehydes. It plays an important role in the enantioselective synthesis of the core ring skeleton of leucosceptroids A-D and in steroselective sulfoxidation.
Chemical properties
white to off-white crystalline powder
The Uses of N-Phenyl-bis(trifluoromethanesulfonimide)
N-Phenylbis(trifluoromethanesulfonamide) is used in the enantioselective synthesis of β-amino acids via the Mannich reaction. Used in the synthesis of sphingosine 1-phosphate-1 receptor agonists usefu l in pharmaceutical application.
The Uses of N-Phenyl-bis(trifluoromethanesulfonimide)

To a solution of the SM (798 mg, 3.14 mmol) in DCM (7 mL) was added DMAP (38.3 mg, 0.314 mmol), TEA (1.31 mL, 9.41 mmol), and Tf2NPh (1.68 g, 4.71 mmol). The reaction mixture was stirred at RT for 90 min, after which time it was concentrated in vacuo. The resulting material was purified by Prep LC (50 g silica, 0-20% EtOAc/heptane) to provide the product as a yellow oil which crystallized upon standing. [1.17 g, 97%]
The Uses of N-Phenyl-bis(trifluoromethanesulfonimide)
N-Phenylbis(trifluoromethanesulfonamide) is used in the enantioselective synthesis of β-amino acids via the Mannich reaction. Used in the synthesis of sphingosine 1-phosphate-1 receptor agonists useful in pharmaceutical application.
The Uses of N-Phenyl-bis(trifluoromethanesulfonimide)
Acts as a transparent strong electron-withdrawing p-type dopant in carbon nanotubes
Reactant for:
Synthesis of amphoteric alpha-boryl aldehydes
Enantioselective synthesis of core ring skeleton of leucosceptroids A-D
Stereoselective synthesis of monoamine reuptake inhibitor NS9544 acetate
Stereoselective sulfoxidation
Synthesis
Add methylene chloride, aniline, 4-dimethylaminopyridine (DMAP), and triethylamine to a reactor equipped with a thermometer, distillation device, and mechanical stirring. When the vacuum of the reactor is evacuated to -0.095MPa and cooled to -40°C, trifluoromethanesulfonyl fluoride gas is introduced and then stirred at -20°C ~ 0°C and 0.02MPa ~ 0.1MPa reaction pressure, and react for 6 hours Finally, the excess trifluoromethanesulfonyl fluoride gas in the reactor is released and collected by cooling. After the inside of the reactor is brought to normal pressure, 500 mL of water is added, and the liquid is separated. A light yellow solid is obtained after the organic phase is heated to 40°C and methylene chloride is distilled. The light yellow solid is recrystallized with toluene to obtain 650g of white crystal N-Phenyl-bis(trifluoromethanesulfonimide).
Properties of N-Phenyl-bis(trifluoromethanesulfonimide)
| Melting point: | 100-102 °C(lit.) |
| Boiling point: | 305.3±52.0 °C(Predicted) |
| Density | 1.766±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in methanol. Slightly soluble in chloroform and ethyl acetate. |
| form | Crystals or Crystalline Powder |
| pka | -13.12±0.50(Predicted) |
| color | White or colorless |
| Sensitive | Moisture Sensitive |
| BRN | 1269141 |
| Stability: | Moisture Sensitive |
| InChI | InChI=1S/C8H5F6NO4S2/c9-7(10,11)20(16,17)15(6-4-2-1-3-5-6)21(18,19)8(12,13)14/h1-5H |
| CAS DataBase Reference | 37595-74-7(CAS DataBase Reference) |
| NIST Chemistry Reference | N-phenyltrifluoromethanesulfonimide(37595-74-7) |
Safety information for N-Phenyl-bis(trifluoromethanesulfonimide)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-Phenyl-bis(trifluoromethanesulfonimide)
| InChIKey | DIOHEXPTUTVCNX-UHFFFAOYSA-N |
| SMILES | C(F)(F)(F)S(N(C1=CC=CC=C1)S(C(F)(F)F)(=O)=O)(=O)=O |
N-Phenyl-bis(trifluoromethanesulfonimide) manufacturer
JSK Chemicals
GLR Innovations
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 N-Phenylbis(trifluoromethanesulfonimide) CAS 37595-74-7View Details
N-Phenylbis(trifluoromethanesulfonimide) CAS 37595-74-7View Details
37595-74-7 -
 n-Phenyl-bis(trifluoromethanesulphonimide) CAS 37595-74-7View Details
n-Phenyl-bis(trifluoromethanesulphonimide) CAS 37595-74-7View Details
37595-74-7 -
![N-Phenylbis(trifluoromethanesulfonimide) [Triflating Reagent] CAS 37595-74-7](https://img.chemicalbook.in//Content/image/CP5.jpg) N-Phenylbis(trifluoromethanesulfonimide) [Triflating Reagent] CAS 37595-74-7View Details
N-Phenylbis(trifluoromethanesulfonimide) [Triflating Reagent] CAS 37595-74-7View Details
37595-74-7 -
 N-Phenyl-bis(trifluoromethanesulfonimide) 98% CAS 37595-74-7View Details
N-Phenyl-bis(trifluoromethanesulfonimide) 98% CAS 37595-74-7View Details
37595-74-7 -
 N-Phenyl-bis(trifluoromethanesulfonimide) CAS 37595-74-7View Details
N-Phenyl-bis(trifluoromethanesulfonimide) CAS 37595-74-7View Details
37595-74-7 -
 N-Phenyl-bis(trifluoromethanesulfonimide) CAS 37595-74-7View Details
N-Phenyl-bis(trifluoromethanesulfonimide) CAS 37595-74-7View Details
37595-74-7 -
 GLR N-Phenyl-bis(trifluoromethanesulfonimide) , CAS NO-37595-74-7, Grade: Lab Grade, Packaging Size: Gm,KGView Details
GLR N-Phenyl-bis(trifluoromethanesulfonimide) , CAS NO-37595-74-7, Grade: Lab Grade, Packaging Size: Gm,KGView Details
37595-74-7 -
 N-Phenyl-bis(Trifluoromethanesulfonimide), Technical Grade, 900mlView Details
N-Phenyl-bis(Trifluoromethanesulfonimide), Technical Grade, 900mlView Details
37595-74-7
