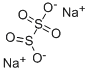
POTASSIUM METABISULFITE
- CAS NO.:16731-55-8
- Empirical Formula: K2O5S2
- Molecular Weight: 222.32
- MDL number: MFCD00167605
- EINECS: 240-795-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
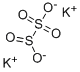
What is POTASSIUM METABISULFITE?
Chemical properties
White to slightly yellowish fine cryst. powder
Chemical properties
Potassium metabisulfite occurs as white or colorless free-flowing crystals, crystalline powder, or granules, usually with an odor of sulfur dioxide.
The Uses of POTASSIUM METABISULFITE
Potassium Metabisulfite is a chemical preservative and antioxi- dant obtained as white or colorless crystals, powder, or granules. it is soluble in water and insoluble in alcohol. the sulfite salt yields sulfurous acid at a low ph. it is used as a food preservative.
The Uses of POTASSIUM METABISULFITE
As antifermentative in breweries and wineries; bleaching straw; preservative for fruits and vegetables.
The Uses of POTASSIUM METABISULFITE
Potassium Metabisulfite (2:1) is used as a chemical preservative.
What are the applications of Application
Potassium disulfite is an inorganic reagent used for research purposes
General Description
Potassium disulfite (Potassium metabisulfite, PMB) is an inorganic salt with antimicrobial properties. It is a sulfiting agent that prevents browning of foods.
Pharmaceutical Applications
Potassium metabisulfite is used in applications similar to those of sodium metabisulfite in pharmaceuticals, and in the food, brewing, and wine making industries. It is used as an antioxidant, antimicrobial preservative and sterilizing agent.
Contact allergens
Potassium metabisulfite is an antioxidant used as an antifermentative agent in breweries and wineries, as a preservative of fruits and vegetables, and to bleach straw. Reactions to both sodium and potassium metabisulfite are expected.
Safety Profile
Experimental reproductive effects. A very irritating material. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and K2SO3. See also SULFITES.
Safety
Potassium metabisulfite is used in a variety of foods and
pharmaceutical preparations, including oral, otic, rectal, and
parenteral preparations. Potassium metabisulfite is considered a
very irritating material, and may cause dermatitis on exposed
skin.
Hypersensitivity reactions to potassium metabisulfite and other
sulfites, mainly used as preservatives in food products, have been
reported. Reactions include bronchospasm and anaphylaxis; some
deaths have also been reported, especially in those with a history of
asthma or atopic allergy.These reactions have led to
restrictions by the FDA on the use of sulfites in food applications.
However, this restriction has not been extended to their use in
pharmaceutical applications. Indeed, epinephrine (adrenaline)
injections used to treat severe allergic reactions may contain
sulfites.
The WHO has set an acceptable daily intake of sulfites, as SO2,
at up to 0.35 mg/kg body-weight.
storage
Potassium metabisulfite should be stored in a cool, dark place.
When stored at a maximum temperature of 25°C and maximum
relative humidity of 45%, the shelf-life is 6 months. Potassium
metabisulfite decomposes at temperatures above 150°C. In the air, it
oxidizes to the sulfate, more readily in the presence of moisture.
In aqueous solution, potassium metabisulfite forms potassium
bisulfite (KHSO3) which exerts a strong reducing effect.
Incompatibilities
Potassium metabisulfite is incompatible with strong acids, water,
and most common metals. It reacts with nitrites and sodium nitrate
at room temperature, which occasionally results in the formation of
flame. The reaction may be explosive if water is present. Potassium
metabisulfite liberates SO2 with acids.
Sulfites, including potassium metabisulfite, can react with
various pharmaceutical compounds including sympathomimetics
such as epinephrine (adrenaline),chloramphenicol,cisplatin,
and amino acids,which can result in their pharmacological
inactivation. Sulfites are also reported to react with phenylmercuric
nitrate,and may adsorb onto rubber closures.
Regulatory Status
GRAS listed. Accepted in Europe for use as a food additive in certain applications. Included in the FDA Inactive Ingredients Database (IM and IV injection; otic and rectal solutions and suspensions). Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Properties of POTASSIUM METABISULFITE
| Melting point: | 190°C |
| Density | 2.34 g/cm3 |
| vapor density | 2.3 (vs air) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.1 M at 20 °C, clear, colorless |
| form | Powder/Solid |
| color | White to slightly yellow |
| Specific Gravity | 2.3 |
| PH Range | 3-4.5 at 22.2 g/l at 25 °C |
| Odor | Odorless |
| PH | 2.5-4.5 (25℃, 0.1M in H2O) |
| Water Solubility | Soluble in water. Insoluble in ethanol. |
| Sensitive | Air Sensitive |
| Decomposition | 150°C |
| λmax | 267nm(H2O)(lit.) |
| Merck | 14,7645 |
| EPA Substance Registry System | Disulfurous acid, potassium salt (1:2) (16731-55-8) |
Safety information for POTASSIUM METABISULFITE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for POTASSIUM METABISULFITE
POTASSIUM METABISULFITE manufacturer
H. K. Group
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 Potassium metabisulfite 98%View Details
Potassium metabisulfite 98%View Details -
 Potassium metabisulfite 98%View Details
Potassium metabisulfite 98%View Details -
 Potassium metabisulfite CAS 16731-55-8View Details
Potassium metabisulfite CAS 16731-55-8View Details
16731-55-8 -
 Potassium metabisulfite CAS 16731-55-8View Details
Potassium metabisulfite CAS 16731-55-8View Details
16731-55-8 -
 Potassium metabisulfite CAS 16731-55-8View Details
Potassium metabisulfite CAS 16731-55-8View Details
16731-55-8 -
 Potassium metabisulphite, GR 98%+ CAS 16731-55-8View Details
Potassium metabisulphite, GR 98%+ CAS 16731-55-8View Details
16731-55-8 -
 Potassium disulfite CAS 16731-55-8View Details
Potassium disulfite CAS 16731-55-8View Details
16731-55-8 -
 Potassium disulfite CAS 16731-55-8View Details
Potassium disulfite CAS 16731-55-8View Details
16731-55-8
