Benzoic acid
Synonym(s):Acidum benzoicum;Benzoic acid;Benzoic acid solution;Phenylformic acid, Benzene carboxylic acid
- CAS NO.:65-85-0
- Empirical Formula: C7H6O2
- Molecular Weight: 122.12
- MDL number: MFCD00002398
- EINECS: 200-618-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
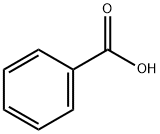
What is Benzoic acid?
Description
Benzoic acid is a colorless, crystalline solid also known as benzenecarboxylic acid. It is the simplest aromatic carboxylic acid, with a carboxyl group (-COOH) bonded directly to the benzene ring. It is found naturally in the benzoin resin of a number of plants.
Chemical properties
Benzoic acid,C6H5COOH, also known as benzene carboxylic acid and phenyl formic acid,is a colorless, monoclinic crystalline solid that has a melting point of 122.4"C and sublimes readily at 100·C. It is an aromatic carboxylic acid that is slightly soluble in water and moderately soluble in alcohol and ether.
Physical properties
Colorless to white needles, scales, or powder with a faint benzoin or benzaldehyde-like odor. Shaw et al. (1970) reported a taste threshold in water of 85 ppm.
Occurrence
Reported found in fresh apple, apricot (Prunus armeniaca L.), strawberry fruit, cherry (Prunus cerasus L.), butter, boiled and cooked beef, pork fat, white wine, black tea, green tea, fresh plum, mushroom, Bourbon vanilla (Vanilla planifolia Andrews), and other natural sources. Reported as being a constituent of various oils, resins and flower absolutes; hyacinth, tuberose, neroli bigarade, Chinese cinnamon, cinnamon leaves, anise, vertiver, ylang-ylang, Tolu balsam and clove; it is contained in fairly sizable amounts in gum benzoin, from which benzoic acid is extracted by sublimation.
History
Benzoic acid was first isolated from the dry distillation of benzoin by Blaise de Vigenère (1523–1596) in the 16th century. Friedrich W?hler (1800–1882) and Justus von Liebig (1803–1873) prepared benzoic acid from oxidizing bitter almond oil (benzaldehyde) in 1832 and determined the formula for each of these compounds. They proposed that bitter almond oil, C7H6O, and benzoic acid were derivatives from the benzoyl radical, C7H5O; the radical theory was a major early theory in the development of organic chemistry.
The Uses of Benzoic acid
Benzoic acid is a simple organic compound employed as a preservative. Benzoic acid and sodium benzoate (C6H5COONa) are used as food preservatives and added to foods, juices, and beverages that are acidic. It also is often found in topical antifungal preparations.
The Uses of Benzoic acid
Calorimetry
Benzoic acid is the most commonly used chemical standard to determine the heat of capacity of a bomb calorimeter.
Feed stock
Benzoic acid is used to make a large number of chemicals, important examples of which are :
Benzoyl chloride, C6H5C(O)Cl, is obtained by treatment of benzoic with thionyl chloride, phosgene or one of the chlorides of phosphorus. C6H5C(O) Cl is an important starting material for several benzoic acid derivates like benzyl benzoate, which is used in artificial flavours and insect repellents.
Food preservative
Benzoic acid and its salts are used as a food preservatives, represented by the E-numbers E210 , E211 , E212 , and E213 . Benzoic acid inhibits the growth of mold, yeast and some bacteria. It is either added directly or created from reactions with its sodium, potassium, or calcium salt. The mechanism starts with the absorption of benzoic acid in to the cell.
Medicinal
Benzoic acid is a constituent of Whitfiel's ointment which is used for the treatment of fungal skin diseases such as tinea, ringworm, and athlete's foot. As the principal component of benzoin resin, benzoic acid is also a major ingredient in both tincture of benzoin and Fria's balsam. Such products have a long history of use as topical antiseptics and inhalant decongestants.
Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century.
Background
As a fungistatic compound Benzoic acid is widely used as a food preservative. It is conjugated to GLYCINE in the liver and excreted as hippuric acid. As the sodium salt form, sodium benzoate is used as a treatment for urea cycle disorders due to its ability to bind amino acids. This leads to excretion of these amino acids and a decrease in ammonia levels. Recent research shows that sodium benzoate may be beneficial as an add-on therapy (1 gram/day) in schizophrenia. Total Positive and Negative Syndrome Scale scores dropped by 21% compared to placebo.
Definition
ChEBI: A compound comprising a benzene ring core carrying a carboxylic acid substituent.
Production Methods
Industrial preparations
Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. The process is catalyzed by cobalt or manganese naphthenates. The process uses cheap raw materials, proceeds in high yield, and is considered environmentally green.
Laboratory synthesis
Benzoic acid is cheap and readily available, so the laboratory synthesis of benzoic acid is mainly practiced for its pedagogical value. It is a common undergraduate preparation.
For all syntheses, benzoic acid can be purified by recrystallization from water because of its high solubility in hot water and poor solubility in cold water. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe. Other possible recrystallization solvents include acetic acid (anhydrous or aqueous), benzene, acetone, petroleum ether, and a mixture of ethanol and water. The solubility of benzoic acid in over 40 solvents with references to original sources can be found as part of the Open Notebook Science Challenge.
Preparation
Benzoic acid is synthesized by oxidation of toluene with nitric acid or sodium bichromate or from benzonitrile.
Reactions
Reactions of benzoic acid can occur at either the aromatic ring or the carboxyl group :
Aromatic ring
Electrophilic aromatic substitution reaction will take place mainly in 3- position due to the electron-withdrawing carboxylic group; i.e. benzoic acid is meta directing.
The second substitution reaction (on the right) is slower because the first nitro group is deactivating. Conversely, if an activating group (electron - donating) was introduced (e.g., alkyl), a second substitution reaction would occur more readily than the first and the disubstituted product might accumulate to a significant extent.
Carboxyl group
All the reactions mentioned for carboxylic acids are also possible for benzoic acid.
Benzoic acid esters are the product of the acid catalysed reaction with alcohols. Benzoic acid amides are more easily available by using activated acid derivatives (such as benzoyl chloride) or by coupling reagents used in peptide synthesis like DCC and DMAP.
The more active benzoic anhydride is formed by dehydration using acetic anhydride or phosphorus pentoxide.
Highly reactive acid derivatives such as acid halides are easily obtained by mixing with halogenation agents like phosphorus chlorides or thionyl chloride.
Ortho esters can be obtained by the reaction of alcohols under acidic water free conditions with benzonitrile.
Reduction to benzaldehyde and benzyl alcohol is possible using DIBAL- H , Li Al H4 or sodium boro hydride.
The copper catalyzed decarboxylation of benzoate to benzene may be effected by heating in quinoline. Also, Hunsdiecker decarboxylation can be achieved by forming the silver salt and heating. Benzoic acid can also be decarboxylated by heating with an alkali hydroxide or calcium hydroxide.
Biotechnological Production
Benzoic acid is exclusively chemically synthesized on an industrial scale. Toluene from petrochemical routes is oxidized in the presence of the catalyst potassium permanganate to benzoic acid . However, a recent study described for the first time a benzoic acid production process by fermentation using Streptomyces maritimus. The production of benzoic acid during cultivation on glucose, starch, and cellobiose has been investigated. The best results have been achieved with product concentrations of 460 mg.L-1 in 6 days using starch as substrate. Additionally, a genetically modified S. maritimus optimized for endo-glucanasesecretion has been tested on phosphoric acid swollen cellulose. A final product concentration of 125 mg.L-1 was observed after 4 days of cultivation.
Aroma threshold values
85 ppm.
Synthesis Reference(s)
Canadian Journal of Chemistry, 50, p. 3741, 1972 DOI: 10.1139/v72-592
Chemistry Letters, 5, p. 147, 1976
Tetrahedron Letters, 23, p. 2347, 1982 DOI: 10.1016/S0040-4039(00)87338-4
Air & Water Reactions
Vapor from molten Benzoic acid may form explosive mixture with air. The finely powdered dry acid is a significant dust explosion hazard [Bretherick, 5th ed., 1995, p. 884]. In air very rapid combustion occurs [Wilson, L.Y. et al., J. Chem. Ed., 1985, 62(10), p. 902]. Slightly soluble in water.
Reactivity Profile
At high temperature Benzoic acid can react with oxidizing reagents.
Hazard
Moderately toxic by ingestion. Use restricted to 0.1% in foods.
Health Hazard
Dust may be irritating to nose and eyes. At elevated temperatures, fumes may cause irritation of eyes, respiratory system, and skin.
Fire Hazard
Behavior in Fire: Vapor from molten Benzoic acid may form explosive mixture with air. Concentrated dust may form explosive mixture.
Agricultural Uses
Fungicide, Insecticide: Used in the manufacture of benzoates; plasticizers, benzoyl chloride, alkyd resins, in the manufacture of food preservatives, in use as a dye binder in calico printing; in curing of tobacco, flavors, perfumes, dentifrices, standard in analytical chemistry. Not currently registered for use in the U.S. Benzoic acid is currently used in about a dozen European countries.
Pharmaceutical Applications
Benzoic acid is widely used in cosmetics, foods, and pharmaceuticals, as an antimicrobial preservative. Greatest
activity is seen at pH values between 2.5–4.5.
Benzoic acid also has a long history of use as an antifungal
agent in topical therapeutic preparations such as Whitfield’s
ointment (benzoic acid 6% and salicylic acid 3%).
Trade name
RETARDER BA®; MICROL® Preservative; TENN-PLAS®; RETARDEX®; SALVO LIQUID®; SALVO POWDER®; TULSA®
Side Effects
One of the most commonly reported side effects of benzoic acid is skin irritation. This can range from mild redness and itching to more severe dermatitis in sensitive individuals.
Another significant side effect is respiratory irritation. Inhalation of benzoic acid dust or fumes can lead to coughing, sneezing, and even asthma-like symptoms in susceptible individuals.
Benzoic acid can also cause eye irritation. Ingesting large amounts of benzoic acid can lead to gastrointestinal issues.
Additionally, benzoic acid can cause allergic reactions in some individuals.
Safety
Ingested benzoic acid is conjugated with glycine in the liver to yield
hippuric acid, which is then excreted in the urine; care should be
taken when administering benzoic acid to patients with chronic liver
disease. Benzoic acid is a gastric irritant, and a mild irritant to the
skin. It is also a mild irritant to the eyes and mucous
membranes. Allergic reactions to benzoic acid have been
reported, although a controlled study indicated that the incidence
of urticaria in patients given benzoic acid is no greater than in those
given a lactose placebo. It has been reported that asthmatics may
become adversely affected by benzoic acid contained in some
antiasthma drugs.
The WHO acceptable daily intake of benzoic acid and other
benzoates, calculated as benzoic acid, has been set at up to 5 mg/kg
body-weight. The minimum lethal human oral dose of benzoic
acid is 500 mg/kg body-weight.
LD50 (cat, oral): 2 g/kg
LD50 (dog, oral): 2 g/kg
LD50 (mouse, IP): 1.46 g/kg
LD50 (mouse, oral): 1.94 g/kg
LD50 (rat, oral): 1.7 g/kg
Potential Exposure
Benzoic acid is used in production of plasticizers, benzoyl chloride, sodium benzoate and alkyl resins; in the manufacture of benzoates; in the manufacture of food preservatives; as a dye binder in calico printing; in curing of tobacco, flavors, perfumes, dentifrices; standard in analytical chemistry; antifungal agent.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately.If this chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
Benzoic acid was not genotoxic in bacterial assays or in in vitro mammalian assays.
Source
Naturally occurs in cranberries, ligonberries (1,360 ppm), peppermint leaves (20–200 ppb), tea leaves, cassia bark, carob, blessed thistle, purple foxglove, jasmine, hyacinth, apples, tobacco leaves, daffodils, autumn crocus, prunes, anise seeds, ripe cloves, and wild black cherry tree bark (Duke, 1992; quoted, Verschueren, 1983).
Schauer et al. (1999) reported benzoic acid in diesel fuel at a concentration of 1,260 μg/g. Identified as an oxidative degradation product in the headspace of a used engine oil (10–30W) after 4,080 miles (Levermore et al., 2001).
The gas-phase tailpipe emission rate from California Phase II reformulated gasoline-powered automobile equipped with a catalytic converter was 124 μg/km (Schauer et al., 2002). Benzoic acid is a by-product of benzoyl peroxide used in the bleaching of freshly milled wheat flour.
Environmental Fate
Biological. Benzoic acid may degrade to catechol if it is the central metabolite whereas, if protocatechuic acid (3,4-dihydroxybenzoic acid) is the central metabolite, the precursor is 3- hydroxybenzoic acid (Chapman, 1972). Other compounds identified following degradation of benzoic acid to catechol include cis,cis-muconic acid, (+)-muconolactone, 3-oxoadipate enol lactone, and 3-oxoadipate (quoted, Verschueren, 1983).
Photolytic. Titanium dioxide suspended in an aqueous solution and irradiated with UV light (λ = 365 nm) converted benzoic acid to carbon dioxide at a significant rate (Matthews, 1986). An aqueous solution containing chlorine and irradiated with UV light (λ = 350 nm) converted benzoic acid to salicylaldehyde and unidentified chlorinated compounds (Oliver and Carey, 1977).
Production
Sodium benzoate is an important benzoic acid derivative produced industrially by neutralization of benzoic acid using sodium hydroxide or sodium bicarbonate solution. Calcium benzoate, potassium benzoate, and other benzoate salts are also produced. Its first industrial synthesis was the hydrolysis of benzotrichloride to calcium benzoate, followed by acidification. This method has been completely displaced by the air oxidation of toluene, which avoids the problem of product contamination with chlorinated byproducts.
Storage
Aqueous solutions of benzoic acid may be sterilized by autoclaving or by filtration.
A 0.1% w/v aqueous solution of benzoic acid has been reported to be stable for at least 8 weeks when stored in polyvinyl chloride bottles, at room temperature.
The bulk material should be stored in a well-closed container in a cool, dry place.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.
Purification Methods
For use as a volumetric standard, analytical reagent grade benzoic acid should be carefully fused to ca 130o (to dry it) in a platinum crucible, and then powdered in an agate mortar. Benzoic acid has been crystallised from boiling water (charcoal), aqueous acetic acid, glacial acetic acid, *C6H6, aqueous EtOH, pet ether (b 60-80o), and from EtOH solution by adding water. It is readily purified by fractional crystallisation from its melt and by sublimation in a vacuum at 80o. The S-benzylisothiuronium salt has m 167o (from EtOH/H2O). [Beilstein 9 IV 273.]
Incompatibilities
Benzoic acid is Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, caustics, ammonia, amines, isocyanates. Dust forms an explosive mixture with air.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM and IV injections, irrigation solutions, oral solutions, suspensions, syrups and tablets, rectal, topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Benzoic acid
| Melting point: | 121-125 °C(lit.) |
| Boiling point: | 249 °C(lit.) |
| Density | 1.08 |
| vapor density | 4.21 (vs air) |
| vapor pressure | 10 mm Hg ( 132 °C) |
| refractive index | 1.504 |
| FEMA | 2131 | BENZOIC ACID |
| Flash point: | 250 °F |
| storage temp. | 2-8°C |
| solubility | soluble, clear, colorless (95% ethanol, 1gm/3mL) |
| form | Solid |
| appearance | white crystals |
| pka | 4.19(at 25℃) |
| color | White to yellow-beige to orange |
| PH | 3.66(1 mM solution);3.12(10 mM solution);2.6(100 mM solution); |
| Odor | at 100.00 %. faint balsam urine |
| Water Solubility | Slightly soluble. 0.34 g/100 mL |
| Merck | 14,1091 |
| JECFA Number | 850 |
| BRN | 636131 |
| Henry's Law Constant | (x 10-8 atm?m3/mol):
7.02 (calculated, U.S. EPA, 1980a) |
| Stability: | Stable. Combustible. Incompatible with strong bases, strong oxidizing agents, alkalies. |
| CAS DataBase Reference | 65-85-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid(65-85-0) |
| EPA Substance Registry System | Benzoic acid (65-85-0) |
Safety information for Benzoic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Benzoic acid
| InChIKey | WPYMKLBDIGXBTP-UHFFFAOYSA-N |
Benzoic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Benzoic Acid 98%View Details
Benzoic Acid 98%View Details -
 BENZOIC ACID 99%View Details
BENZOIC ACID 99%View Details -
 BENZOIC ACID I.P. 99%View Details
BENZOIC ACID I.P. 99%View Details -
 BENZOIC ACID 99%View Details
BENZOIC ACID 99%View Details -
 Benzoic acid 99%View Details
Benzoic acid 99%View Details -
 Benzoic acid 98%View Details
Benzoic acid 98%View Details -
 Benzoic Acid CASView Details
Benzoic Acid CASView Details -
 Benzoic Acid CASView Details
Benzoic Acid CASView Details
