POTASSIUM SULFIDE
Synonym(s):Potassium monosulfide;Potassium sulfide
- CAS NO.:1312-73-8
- Empirical Formula: K2S
- Molecular Weight: 110.26
- MDL number: MFCD00043083
- EINECS: 215-197-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:56
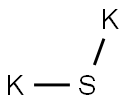
What is POTASSIUM SULFIDE?
Chemical properties
colourless solid; turns brownish-red on
Chemical properties
Potassium sulfide is a brownish-red crystalline solid.
The Uses of POTASSIUM SULFIDE
Potassium Sulfide is reported to be used as a reactant for the efficient synthesis of variously substituted thiophenes through copper-catalyzed tandem S-alkenylation with 1,4-diiodo-1,3-dienes. It is also used as a reactant for the synthesis of KBi6.33S10 and K2Bi8S13 at high temperature.
The Uses of POTASSIUM SULFIDE
Reagent in analytical chemistry, depilatory, medicine.
Production Methods
Potassium sulfide, K2S, yellowish to reddish solid, soluble, formed by heating potassium sulfate and carbon to a high temperature; potassium hydrogen sulfide, potassium bisulfide, potassium acid sulfide KHS, formed in solution by reaction of potassium hydroxide or carbonate solution and excess H2S.
General Description
A red crystalline solid. Denser than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion.
Air & Water Reactions
May spontaneously ignite with exposure to air. Deliquescent. Water soluble
Reactivity Profile
A reducing agent. So readily oxidized as to be pyrophoric in air [Bretherick 1979 p. 120]. POTASSIUM SULFIDE is incompatible with chloroform and nitrogen oxide.
Hazard
Flammable, dangerous fire risk, may ignite spontaneously, explosive in the form of dust or pow- der.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
Poison by ingestion and inhalation. Emits H2S in contact with acids; steam. A flammable solid. Unstable; may explode on percussion or rapid heating. Ignites on contact with nitrogen oxide. Reacts with H2O to form KOH and KSH. When heated to decomposition it emits very toxic fumes of K2O and SOx. See also SULFIDES.
Potential Exposure
Potassium sulfide is used as a reagent in analytical chemistry; and in pharmaceutical preparations.
Shipping
UN1382 Potassium sulfide, anhydrous or Potassium sulfide with <30% water of crystallization, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material.
Incompatibilities
May explosively decompose from shock, friction, or concussion. Dust or granules may spontaneously ignite on contact with air. The aqueous solution is a strong base; reacts violently with strong acids and acid fumes. The solid material decomposes on contact with acids producing hydrogen sulfide, and oxidizers producing sulfur dioxide.
Properties of POTASSIUM SULFIDE
| Melting point: | 912° |
| Boiling point: | 912 °C |
| Density | 1.74 |
| solubility | soluble in H2O, ethanol; insoluble in ethyl ether |
| form | Powder |
| color | white to yellow |
| Water Solubility | very soluble H2O, alcohol, glycerol; insoluble ether [MER06] |
| Sensitive | air sensitive, hygroscopic |
| Stability: | Stable, but air-sensitive. Flammable. Contact with acids liberates poisonous hydrogen sulfide. Anhydrous material may be spontaneously combustible. |
| CAS DataBase Reference | 1312-73-8(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium sulfide (K2S) (1312-73-8) |
Safety information for POTASSIUM SULFIDE
Computed Descriptors for POTASSIUM SULFIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 1312-73-8 Potassium sulfide 98%View Details
1312-73-8 Potassium sulfide 98%View Details
1312-73-8 -
 Potassium sulphide CAS 1312-73-8View Details
Potassium sulphide CAS 1312-73-8View Details
1312-73-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
