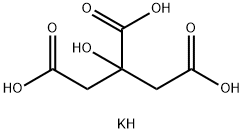
Potassium Citrate
Synonym(s):Citric acid tripotassium salt;Potassium citrate tribasic monohydrate;Tripotassium citrate
- CAS NO.:866-84-2
- Empirical Formula: C6H9KO7
- Molecular Weight: 232.23
- MDL number: MFCD00036362
- EINECS: 212-755-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:02
What is Potassium Citrate?
Toxicity
LD50 (dog): Intravenous 176 mg/kg.
Description
Potassium citrate is a potassium salt of citric acid with the molecular formula C6H5K3O7. It is a white, slightly hygroscopic crystalline powder. It is odorless with a saline taste.
As a food additive, potassium citrate is used to regulate acidity and is known as E number E332. Medicinally, it may be used to control kidney stones derived from either uric acid or cystine.
Chemical properties
Colorless or white crystals or powder; cooling, saline taste; odorless; deliquescent. Soluble in water and glycerol; almost insoluble in alcohol.
The Uses of Potassium Citrate
potassium citrate is it acts as a buffer, maintaining product pH constant, a pH adjuster and a product stabilizer. This is a potassium salt.
The Uses of Potassium Citrate
For use as an electrolyte replenisher and in the treatment of hypokalemia.
The Uses of Potassium Citrate
Potassium citrate is rapidly absorbed when given by mouth and is excreted in the urine. Since it is an alkaline salt it is effective in reducing the pain and frequency of urination when these are caused by highly acidic urine . It is used for this purpose in dogs and cats, but is chiefly employed as a non-irritating diuretic.
Potassium citrate is an effective way to treat/manage gout and arrhythmia, if the patient is hypokalemic.
It is widely used to treat urinary calculi (kidney stones) and is often used by patients with cystinuria .
What are the applications of Application
Potassium citrate tribasic solution is a buffering agent and chelator
Background
Potassium citrate (also known as tripotassium citrate) is a potassium salt of citric acid. It is a white, hygroscopic crystalline powder. It is odorless with a saline taste. It contains 38.3% potassium by mass. In the monohydrate form it is highly hygroscopic and deliquescent. Potassium citrate is used to treat a kidney stone condition called renal tubular acidosis. Potassium Citrate is indicated also for the management of Hypocitraturic calcium oxalate nephrolithiasis.
Indications
For the management of renal tubular acidosis, hypocitraturic calcium oxalate nephrolithiasis, and uric acid lithiasis with or without calcium stones.
Definition
ChEBI: The anhydrous form of the tripotassium salt of citric acid.
Production Methods
Potassium citrate is produced by adding potassium bicarbonate or potassium carbonate to a solution of citric acid until effervescence ceases, filtering the solution and evaporating to granulation.
Flammability and Explosibility
Non flammable
Industrial uses
Potassium citrate is used in the United Kingdom as an over-the-counter drug for the relief from discomfort experienced in mild urinary-tract infections by increasing the urinary pH. It should be not given to men if they experience pain in the kidney area (risk of kidney stones) or if blood or pus is present in the urine. Also, patients with raised blood pressure or diabetes should avoid taking potassium citrate without consultation with their general practitioner (GP). Caution is generally advised to patients with renal impairment, cardiac problems and the elderly [3a].
Pharmacokinetics
Potassium citrate induces changes in the urine which renders urine less susceptible to the formation of crystals and stones from salts e.g. calcium oxalate, calcium phosphate and uric acid. Increased citrate levels in the urine will make complexation with calcium which decrease the calcium ion activity and decrease the chance for the formation of calcium phosphate crystals. Citrate also inhibits the spontaneous nucleation of calcium oxalate and calcium phosphate.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of K2O.
Metabolism
Potassium Citrate is absorbed and the citrate is metabolised to bicarbonate.
Precautions
Potassium citrate is usually administered by mouth in dilute aqueous solution. This is because of its somewhat caustic effect on the stomach lining, and the potential for other mild health hazards.
In states where non-prescription potassium citrate is legal, the maximum allowable over-the-counter (OTC) dose for elemental potassium is regulated by the FDA to be no more than 100 mg (approximately 3 % of the daily allowance ) . Pure potassium citrate contains 38.28 % potassium.
Properties of Potassium Citrate
| Melting point: | decomposes at 230℃ [KIR78] |
| Density | 1.187 |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| PH | 8.59(1 mM solution);8.9(10 mM solution);9.01(100 mM solution);7.47(1000 mM solution) |
| Odor | at 100.00?%. odorless |
| Water Solubility | 60.91 g/100g saturated solution in water (25°C) [MER06] |
| λmax | λ: 260 nm Amax: 0.045 λ: 280 nm Amax: 0.025 |
| InChI | InChI=1S/C6H8O7.K.H/c7-3(8)1-6(13,5(11)12)2-4(9)10;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);; |
| CAS DataBase Reference | 866-84-2(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, tripotassium salt (866-84-2) |
Safety information for Potassium Citrate
Computed Descriptors for Potassium Citrate
| InChIKey | JOSYFFAWJFRGHM-UHFFFAOYSA-N |
| SMILES | C(O)(C(=O)O)(CC(=O)O)CC(=O)O.[KH] |
Potassium Citrate manufacturer
New Alliance Fine Chem Private Limited
Buradon Inc.
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 POTASSIUM CITRATE 98%View Details
POTASSIUM CITRATE 98%View Details -
 Potassium Citrate 99%View Details
Potassium Citrate 99%View Details -
 Potassium Citrate Cas No 6100-05-6 SPIL, 99.9%, 99.9%View Details
Potassium Citrate Cas No 6100-05-6 SPIL, 99.9%, 99.9%View Details
6100-05-6 -
 Potassium Citrate, Packaging Type: Hdpe Bag, Packaging Size: 25KGView Details
Potassium Citrate, Packaging Type: Hdpe Bag, Packaging Size: 25KGView Details
866-84-2 -
 Potassium Citrate (6100-05-6)View Details
Potassium Citrate (6100-05-6)View Details
6100-05-6 -
 Potassium Citrate - IP/BP/USPView Details
Potassium Citrate - IP/BP/USPView Details
6100-05-6 -
 Potassium Citrate LRView Details
Potassium Citrate LRView Details
6100-05-6 -
 Potassium CitrateView Details
Potassium CitrateView Details
6100-05-6
