Potassium citrate monohydrate
Synonym(s):Citric acid tripotassium salt;Tripotassium citrate;tri-Potassium citrate monohydrate;Tripotassium citrate, Citric acid potassium salt, 2-Hydroxy-1,2,3-Propanetriccarboxylic Acid
- CAS NO.:6100-05-6
- Empirical Formula: C6H11KO8
- Molecular Weight: 250.24
- MDL number: MFCD00150442
- EINECS: 612-062-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 10:19:20
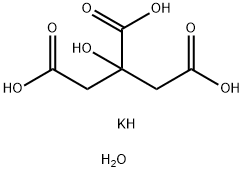
What is Potassium citrate monohydrate?
Chemical properties
White/clear crystalline powder
Chemical properties
Transparent prismatic crystals or a white, granular powder. Potassium citrate is hygroscopic and odorless, and has a cooling, saline taste.
The Uses of Potassium citrate monohydrate
Tripotassium Citrate Monohydrate is used in manufacturing method of printed circuit board for autonomous vehicle to perform recognition, judgment, determination and execution.Citric acid is a weak organic acid that is known as a commodity chemical, as more than a million tonnes are produced every year by mycological fermentation on an industrial scale using crude sugar solutions, such as molasses and strains of Aspergillus niger. Citric acid is widely distributed in plants and in animal tissues and fluids and exist in greater than grace amounts in variety of fruits and vegetables, most notably in citrus fruits such as lemon and limes. Citric acid is mainly used as an acidifier, flavoring agent and chelating agent. Citric acid is also a metabolite of Dimethyl Fumarate (D464965), a compound
The Uses of Potassium citrate monohydrate
In foods and beverages as buffering, sequestering or emulsifying agent.
The Uses of Potassium citrate monohydrate
Potassium Citrate, Monohydrate is a sequestrant and buffer that exists as crystals or powder. it is slightly hygroscopic and possesses the advantageous properties of citric acid without having its acid reaction. a 1% solution has a ph of 7.5–9.0. it reacts with metal ions such as calcium, magnesium, and iron to form a complex. it is soluble in water with a solubility of 1.8 g in 1 ml of 20°c water and 2 g in 1 ml of 80°c water. it is found in artificially sweetened jelly and in certain milk and meat products. uses include processed cheese, pud- dings, and dietetic foods in which sodium is undesirable. it is also termed tripotassium citrate.
What are the applications of Application
Potassium citrate tribasic monohydrate is a buffering agent and chelator
Definition
ChEBI: A hydrate that is the monohydrate form of potassium citrate.
Production Methods
Potassium citrate is prepared by adding either potassium bicarbonate or potassium carbonate to a solution of citric acid until effervescence ceases. The resulting solution is then filtered and evaporated to dryness to obtain potassium citrate.
General Description
Potassium citrate tribasic monohydrate (KCTM) is a potassium salt that has been reported to be efficient in forming aqueous two-phase system (ATPS) with UCON 50-HB-5100, a random copolymer. This product is a high quality pharmacopoeia product that meets the testing specifications of USP (United States Pharmacopoeia). It can be employed in research and pilot studies.
Pharmaceutical Applications
Potassium citrate is used in beverages, foods, and oral pharmaceutical formulations as a buffering and alkalizing agent. It is also used as a sequestering agent and as a therapeutic agent to alkalinize the urine and to relieve the painful irritation caused by cystitis.
Biochem/physiol Actions
Potassium citrate can be used to prepare citrate water that supports the survival of streptozotocin (STZ)-diabetic mice. It might reduce the successive stone formation in calcium nephrolithiasis. Potassium citrate serves as an activator for producing activated carbons.
Side Effects
The most common side effects of potassium citrate are gastrointestinal. Potassium citrate can be irritating to the stomach. Potassium citrate may cause an upset stomach, nausea or vomiting, and diarrhea or loose stools.
Safety
Potassium citrate is used in oral pharmaceutical formulations and is
generally regarded as a nontoxic and nonirritant material by this
route of administration.
Most potassium citrate safety data relate to its use as a
therapeutic agent, for which up to 10 g may be administered daily,
in divided doses, as a treatment for cystitis. Although there are
adverse effects associated with excessive ingestion of potassium
salts, the quantities of potassium citrate used as a pharmaceutical
excipient are insignificant in comparison to those used therapeutically.
(IV, dog): 0.17 g/kg
Storage
Potassium citrate is a stable, though hygroscopic material, and should be stored in an airtight container in a cool, dry place. It is deliquescent in moist air.
Purification Methods
It solubility in H2O is 154% and it loses H2O at 180o. [Beilstein 3 III 1091.]
Incompatibilities
Aqueous potassium citrate solutions are slightly alkaline and will react with acidic substances. Potassium citrate may also precipitate alkaloidal salts from their aqueous or alcoholic solutions. Calcium and strontium salts will cause precipitation of the corresponding citrates. Potassium citrate is incompatible with strong oxidizing agents.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral solutions and suspensions; topical emulsions and aerosol foams). Included in nonparenteral medicines (cutaneous foams and emulsions; oral liquids, granules, mixtures and soluble tablets; topical liquids, emulsions and mousses) licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Potassium citrate monohydrate
| Melting point: | 275 °C (dec.)(lit.) |
| Density | 1.98 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Granular |
| Specific Gravity | 1.98 |
| color | White crystalline |
| Odor | Odorless |
| PH Range | 8 - 9.5 |
| PH | 7.5-9.0 (25℃, 50mg/mL in H2O) |
| Water Solubility | Soluble in water and glycerol. Practically insoluble in alcoholSoluble in water and glycerol. Insoluble in alcohol |
| Merck | 14,7623 |
| BRN | 3924344 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 6100-05-6(CAS DataBase Reference) |
| EPA Substance Registry System | Tripotassium citrate monohydrate (6100-05-6) |
Safety information for Potassium citrate monohydrate
Computed Descriptors for Potassium citrate monohydrate
| InChIKey | PJAHUDTUZRZBKM-UHFFFAOYSA-K |
Potassium citrate monohydrate manufacturer
99 Exports
Rishi Chemical Works Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Potassium citrate, tribasic 98%View Details
Potassium citrate, tribasic 98%View Details -
 Tripotassium citrate monohydrate CAS 6100-05-6View Details
Tripotassium citrate monohydrate CAS 6100-05-6View Details
6100-05-6 -
 TRI-POTASSIUM CITRATE 99%View Details
TRI-POTASSIUM CITRATE 99%View Details -
 Potassium Citrate Tribasic Monohydrate extrapure AR CAS 6100-05-6View Details
Potassium Citrate Tribasic Monohydrate extrapure AR CAS 6100-05-6View Details
6100-05-6 -
 tri-POTASSIUM CITRATE AR CASView Details
tri-POTASSIUM CITRATE AR CASView Details -
 tri-POTASSIUM CITRATE Extra Pure CASView Details
tri-POTASSIUM CITRATE Extra Pure CASView Details -
 Potassium citrate tribasic monohydrate 99% CAS 6100-05-6View Details
Potassium citrate tribasic monohydrate 99% CAS 6100-05-6View Details
6100-05-6 -
 Tri Potassium Citrate, Industrial GradeView Details
Tri Potassium Citrate, Industrial GradeView Details
6100-05-6
