Manganese sulfate monohydrate
Synonym(s):Manganese(II) sulfate monohydrate;Manganous(II) sulfate hydrate
- CAS NO.:10034-96-5
- Empirical Formula: H2MnO5S
- Molecular Weight: 169.02
- MDL number: MFCD00149159
- EINECS: 600-072-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
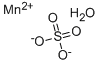
What is Manganese sulfate monohydrate?
Chemical properties
Pale pink crystalline powder, slightly hygroscopic
The Uses of Manganese sulfate monohydrate
Manganese sulfate monohydrate is used to produce manganese by an electrolytic process. The compound is used for dyeing textiles; for producing red glazes on porcelain; in varnish driers; in fertilizers; and in animal feeds to provide manganese as an essential trace element.
The Uses of Manganese sulfate monohydrate
Manganese Sulfate is a source of manganese that functions as a nutrient and dietary supplement. It exists as a powder which is readily soluble in water.
The Uses of Manganese sulfate monohydrate
Manganese(II) sulfate monohydrate is used as a colorant in dyes, fertilizers, animal feeds and red glazes on porcelain. Further, it is used in paints, ceramics, nutrient and dietary supplement. It is involved in the preparation of manganese dioxide. In addition, it serves as a precursor to manganese metal and other manganese compounds.
What are the applications of Application
Manganese(II) sulfate monohydrate is a preparation of manganese for investigations
Preparation
Manganese(II) sulfate is prepared by prolonged heating of any manganese salt with concentrated sulfuric acid. The compound is produced commercially from pyrolusite (MnO2) or rhodochrosite (MnCO3). Either mineral is dissolved in sulfuric acid and the solution evaporated:
MnO2 + H2SO4 → MnSO4 + H2O + ½ O2
Alternatively, manganese dioxide is heated strongly with dehydrated iron(II) sulfate:
2MnO2 + 2FeSO4 → 2MnSO4 + Fe2O3 + ½ O2
Manganese(II) sulfate, prepared by methods involving evaporation of manganese salt with sulfuric acid, is the tetrahydrate, MnSO4•4H2O. The tetrahydrate on gentle heating produces monohydrate, MnSO4•H2O.
Also, manganese(II) sulfate is a by-product in the manufacture of hydroquinone. The process involves reaction of aniline with manganese dioxide in sulfuric acid, followed by the removal of quinone by steam distillation under vacuum.
2C6H5NH2 (aniline) + 5H2SO4 + 4MnO2 → 2C6H4O2 + 4MnSO4 + 2C6H4O2 + 4MnSO4(quinone)
The unreacted acid is neutralized by lime, treated with water, and the solution filtered to separate any excess MnO2 and insoluble residues. Evaporation of the filtrate yields a crude product containing about 80% MnSO4 and 15% (NH4)2SO4.
Manganese(II) sulfate also may be produced by the action of sulfur dioxide with manganese dioxide:
MnO2 + SO2 → MnSO4
Definition
ChEBI: Manganese(II) sulfate monohydrate is a hydrate that is the monohydrate form of manganese(II) sulfate. It has a role as a nutraceutical. It is a hydrate, a manganese molecular entity and a metal sulfate. It contains a manganese(II) sulfate.
General Description
Odorless pale red slightly efflorescent crystals or light pink powder. pH (5% solution) 3.7.
Air & Water Reactions
Water soluble. Hygroscopic.
Reactivity Profile
Manganese(II) sulfate hydrate is incompatible with aluminum and magnesium.
Fire Hazard
Flash point data for Manganese(II) sulfate hydrate are not available; however, Manganese(II) sulfate hydrate is probably combustible.
Biochem/physiol Actions
Manganese sulfate has various industrial applications such as dyeing, porcelain glazing, and the manufacture of fertilizers and boiling oils. MnSO4 is used as a source of manganese ion in biological research, such as in culturing of Bacillus licheniformis and the induction of chromosomal abnormalities in plants. MnSO4 has been utilized to investigate the enzyme dependent glycosylation of endogenous glycoproteins in human skeletal muscle.
Purification Methods
Crystallise it from water (0.9mL/g) at 54-55o by evaporating about two-thirds of the water. It dehydrates above 400o.
Properties of Manganese sulfate monohydrate
| Melting point: | 700 °C |
| Boiling point: | 850 °C |
| Density | 2.95 |
| storage temp. | Store at +15°C to +25°C. |
| solubility | 5-10 g/100 mL at 21°C |
| form | Solid |
| color | Pink |
| PH | 3.0-3.5 (50g/l, H2O, 20℃) |
| Water Solubility | 5-10 g/100 mL at 21 ºC |
| Sensitive | Hygroscopic |
| Merck | 14,5739 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| CAS DataBase Reference | 10034-96-5(CAS DataBase Reference) |
| EPA Substance Registry System | Manganese(II) sulfate monohydrate (10034-96-5) |
Safety information for Manganese sulfate monohydrate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Manganese sulfate monohydrate
| InChIKey | ISPYRSDWRDQNSW-UHFFFAOYSA-L |
Manganese sulfate monohydrate manufacturer
JSK Chemicals
Chemie Trade
Siddhi Impex
JKM Chemtrade
Halogens
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
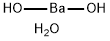
You may like
-
 Manganese(II) sulfate 98%View Details
Manganese(II) sulfate 98%View Details -
 Manganese(II) sulfate monohydrate CAS 10034-96-5View Details
Manganese(II) sulfate monohydrate CAS 10034-96-5View Details
10034-96-5 -
 Manganese(II) sulfate monohydrate CAS 10034-96-5View Details
Manganese(II) sulfate monohydrate CAS 10034-96-5View Details
10034-96-5 -
 Manganese (II) Sulphate Monohydrate pure CAS 10034-96-5View Details
Manganese (II) Sulphate Monohydrate pure CAS 10034-96-5View Details
10034-96-5 -
 Manganese (II) Sulphate Monohydrate extrapure AR CAS 10034-96-5View Details
Manganese (II) Sulphate Monohydrate extrapure AR CAS 10034-96-5View Details
10034-96-5 -
 Manganese (II) sulphate monohydrate 98% CAS 10034-96-5View Details
Manganese (II) sulphate monohydrate 98% CAS 10034-96-5View Details
10034-96-5 -
 Manganese(II) sulfate monohydrate 98.00% CAS 10034-96-5View Details
Manganese(II) sulfate monohydrate 98.00% CAS 10034-96-5View Details
10034-96-5 -
 Manganese (II) sulphate monohydrate 99% AR CAS 10034-96-5View Details
Manganese (II) sulphate monohydrate 99% AR CAS 10034-96-5View Details
10034-96-5
