Calcium citrate
- CAS NO.:813-94-5
- Empirical Formula: C12H10Ca3O14
- Molecular Weight: 498.43
- MDL number: MFCD00078618
- EINECS: 212-391-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:03
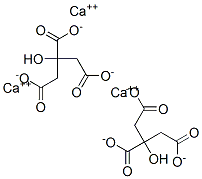
What is Calcium citrate?
Absorption
The percentage of calcium absorbed varies inversely with intake . Tmax of about 3.5-5h varying with formulation .
Toxicity
Patients taking more than 4g of calcium a day are at risk of hypercalcemia and metabolic alkalosis . Chronic intake of calcium supplements is associated with adverse gastrointestinal symptoms such as constipation and flatulence .
Description
Calcium citrate is the calcium salt of citric acid. It is commonly used as a food additive (E333), usually as a preservative, but sometimes for flavor. In this sense, it is similar to sodium citrate. Calcium citrate is also used as a water softener because the citrate ions can chelate unwanted metal ions. Calcium citrate is also found in some dietary calcium supplements (e.g. Citracal). Calcium makes up 21% of calcium citrate by weight.
Chemical properties
white powder or white to colourless crystals
Background
Calcium citrate is a salt typically used as a source of calcium in a variety of over the counter supplements.
Indications
For use as an over the counter calcium supplement.
Production Methods
Calcium citrate is an intermediate in the isolation of citric acid from the fermentation process by which citric acid is produced industrially. The citric acid in the broth solution is neutralized by calcium hydroxide, precipitating insoluble calcium citrate. This is then filtered off from the rest of the broth and washed to give clean calcium citrate.
The calcium citrate thus produced may be sold as-is, or it may be converted to citric acid using dilute sulfuric acid.
Definition
ChEBI: Calcium citrate is an organic calcium salt composed of calcium cations and citrate anions in a 3:2 ratio. It has a role as a nutraceutical, a food additive, a food preservative and a flavouring agent. It contains a citrate(3-).
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Calcium citrate is more easily absorbed (bioavailability is 2.5 times higher than calcium carbonate); it is easier to digest and less likely to cause constipation and gas than calcium carbonate. Calcium citrate can be taken without food and is more easily absorbed than calcium carbonate on an empty stomach. It is also believed that it contributes less to the formation of kidney stones. Calcium citrate consists of around 24% Ca2+, which means that 1000 mg calcium citrate contains around 240 mg Ca2+. The lower Ca2+ content together with the higher price makes it a more expensive treatment option compared to calcium carbonate, but its slightly different application field can justify this.
Biological Activity
In many individuals, bioavailability of calcium citrate is found to be equal to that of the cheaper calcium carbonate.However, alterations to the digestive tract may change how calcium is digested and absorbed. Unlike calcium carbonate, which is basic and neutralizes stomach acid, calcium citrate has no effect on stomach acid. Individuals who are sensitive to antacids or who have difficulty producing adequate stomach acid should choose calcium citrate over calcium carbonate for supplementation. According to recent research into calcium absorption after gastric bypass surgery , calcium citrate may have improved bioavailability over calcium carbonate in Rouxen- Y gastric bypass patients who are taking calcium citrate as a dietary supplement after surgery. This is mainly due to the changes related to where calcium absorption occurs in the digestive tract of these individuals.
Pharmacokinetics
Increases plasma calcium levels leading to a decrease in calcium flux and increase in calcium deposition into bone
Metabolism
Not Available
Properties of Calcium citrate
| solubility | 0.1 M HCl: 0.01 M at 20 °C, clear, colorless |
| Odor | at 100.00?%. odorless |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 813-94-5(CAS DataBase Reference) |
| EPA Substance Registry System | Tricalcium citrate (813-94-5) |
Safety information for Calcium citrate
Computed Descriptors for Calcium citrate
Calcium citrate manufacturer
Nandana Bioscience (OPC) Private Limited
SL Drugs & Pharmaceuticals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Calcium Citrate 98%View Details
Calcium Citrate 98%View Details -
 CALCIUM CITRATE USP 99%View Details
CALCIUM CITRATE USP 99%View Details -
 813-94-5 98%View Details
813-94-5 98%View Details
813-94-5 -
 813-94-5 99%View Details
813-94-5 99%View Details
813-94-5 -
 Calcium citrate CAS 813-94-5View Details
Calcium citrate CAS 813-94-5View Details
813-94-5 -
 Calcium Citrate API MANUFACTURER INDIAView Details
Calcium Citrate API MANUFACTURER INDIAView Details
5785-44-4 -
 White Crystal Solid Calcium Citrate Powder, Packaging Type: DrumView Details
White Crystal Solid Calcium Citrate Powder, Packaging Type: DrumView Details
813-94-5 -
 White Calcium Citrate, 25 kgView Details
White Calcium Citrate, 25 kgView Details
813-94-5
