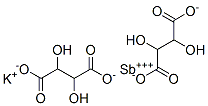
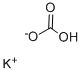
Potassium Bitartrate
Synonym(s):Potassium Bitartrate;Potassium hydrogen tartrate;Monopotassium tartrate;Potassium L -tartrate monobasic;Potassium acid tartrate
- CAS NO.:868-14-4
- Empirical Formula: C4H5KO6
- Molecular Weight: 188.18
- MDL number: MFCD00065392
- EINECS: 212-769-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05
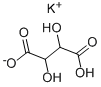
What is Potassium Bitartrate?
Description
L (+)-Potassium hydrogen tartrate (also known as potassium bitartrate) is the potassiumacid salt of tartaric acid (a carboxylic acid). It is the byproduct during the winemaking process. It can be used in baking or as a cleaning solution. When mixed with an acidic liquid such as lemon juice or white vinegar, it can be made of a paste-like cleaning agent for metals or some other cleaning applications. It can also be used as a pH buffer component in chemistry. It has been recently shown that it is capable of treating breast cancer.

Approved by the FDA as a direct food substance, potassium bitartrate is used as an additive, stabilizer, pH control agent, antimicrobial agent, processing aid, or thickener in various food products. Potassium bitartrate has a long history of medical use as a laxative administered as a rectal suppository and is an approved third-class OTC drug in Japan. Potassium bitartrate was one of active ingredients in Phexxi, a non-hormonal contraceptive agent that was approved by the FDA on May 2020.
Chemical properties
Potassium Bitartrate, or tartar, is a salt produced from the crystalsfound on the sides of wine casks. When purified it is known as cream oftartar. It is acid, and is slightly soluble in water.It is used in baking powder, for medicine, and as an acid and buffer in foods.
Chemical properties
Potassium bitartrate, also known as potassium hydrogen tartrate, has formula KC4H5O6, is a byproduct of winemaking. In cooking it is known as cream of tartar. It is the potassium acid salt of tartaric acid, a carboxylic acid.
Occurrence
Potassium bitartrate crystallizes in wine casks during the fermentation of grape juice, and can precipitate out of wine in bottles.The crystals (wine diamonds) will often form on the underside of a cork in wine-filled bottles that have been stored at temperatures below 10°C , and will seldom, if ever, dissolve naturally into the wine.
These crystals also precipitate out of fresh grape juice that has been chilled or allowed to stand for some time. To prevent crystals forming in homemade grape jam or jelly, fresh grape juice should be chilled overnight to promote crystallisation. The potassium bitartrate crystals are removed by filtering through two layers of cheesecloth. The filtered juice may then be made into jam or jelly. In some cases they adhere to the side of the chilled container, making filtering unnecessary.
The crude form (known as beeswing) is collected and purified to produce the white, odorless, acidic powder used for many culinary and other household purposes.
The Uses of Potassium Bitartrate
Largely in baking powders; coloring metals, galvanic tinning of metals; reducer of CrO3 in mordants for wool.
The Uses of Potassium Bitartrate
Potassium L-tartrate monobasic may be used as a reductant during the synthesis of noble metal nanoparticles in the presence of linear polymers as protective agents.
The Uses of Potassium Bitartrate
Potassium hydrogen tartrate [pH(S) = 3.639 / pH(S) = 3.557 (25 °C)] is a certified secondary standard reference buffer used as a calibration buffer standard for pH instruments or pH electrodes.
What are the applications of Application
In food
In food, potassium bitartrate is used for:
Stabilizing egg whites, increasing their heat tolerance and volume
Stabilizing whipped cream, maintaining its texture and volume
Preventing sugar syrups from crystallising
Reducing discolouration of boiled vegetables
Thickening Tartar sauce
Additionally it is used as a component of: Baking powder, as an acid ingredient to activate baking soda Sodium-free salt substitutes, in combination with potassium chloride
A similar acid salt, sodium acid pyrophosphate, can be confused with cream of tartar because of their common function as a component of baking powder.
House hold use
Potassium bitartrate can be mixed with an acidic liquid such as lemon juice or white vinegar to make a paste - like cleaning agent for metals such as brass, aluminum or copper, or with water for other cleaning applications such as removing light stains from porcelain.
This mixture is sometimes mistakenly made with vinegar and sodium bicarbonate (baking soda), which actually react to neutralise each other, creating carbon dioxide and a sodium acetate solution. Cream of tartar was often used in traditional dyeing where the complexing action of the tartrate ions were used to adjust the solubility and hydrolysis of mordant salts such as tin chloride and alum.
Cream of tartar, when mixed into a paste with hydrogen peroxide, can be used to clean rust from some hand tools, notably hand files. The paste is applied and allowed to set for a few hours and then washed off with a baking soda/water solution.
Chemistry
Potassium acid tartrate, also known as potassium hydrogen tartrate, is, according to NIST, used as a primary reference standard for a pH buffer. .
Preparation
potassium bitartrate is mainly produced from the mother liquor of Seignette's salt. These decolorized, purified, and filtered solutions are acidified to pH 3.5 with hydrochloric or sulfuric acid. Since cream of tartar is sparingly soluble, it precipitates and is recovered by centrifugation, dried, and ground before being packaged as fine powder.
General Description
Potassium L-tartrate is found naturally in grapes. Its removal is necessary during wine making as it can precipitate, which is undesirable.
Purification Methods
It crystallises from water (17mL/g) between 100o and 0o. Dry it at 110o. [Beilstein 3 IV 1222.]
References
Coulter, A. D, et al. "Potassium bitartrate crystallisation in wine and its inhibition." Australian Journal of Grape & Wine Research 21.S1(2016):627-641.
Berg, H. W, and M. Akiyoshi. "Utility of potassium bitartrate concentration-product values in wine processing." Amer J Enol Vitic (1971).
Cremer, G. A., and R. Boulton. "The hydrolysis of gelatin by an immobilized acid protease. I. Immobilization and hydrolysis kinetics. " American Journal of Enology & Viticulture 1(1981):14-17.
Dr, María J. Vicent, et al. "The Therapeutic Action of Potassium Bitartrate." Angewandte Chemie 44.26(2005):4061-6.
Properties of Potassium Bitartrate
| Melting point: | 267°C (dec.) |
| Boiling point: | 318℃[at 101 325 Pa] |
| alpha | 32.5 º (c=10, 1N NaOH) |
| Density | 1.954 g/mL at 25 °C(lit.) |
| vapor pressure | 30.69hPa at 25.2℃ |
| Flash point: | 210℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 5.7g/l |
| form | Crystals or Powder |
| color | Colorless opaque or white |
| Specific Gravity | 1.954 |
| PH Range | 3.4 - 3.7 at 20 °C |
| Odor | Odorless |
| PH | 3.4-3.7 (H2O, 20℃)(saturated solution) |
| optical activity | [α]20/D +8.0 to +9.2° |
| Water Solubility | Soluble in water and dilute mineral acid. Insoluble in alcohol. |
| Merck | 14,7615 |
| BRN | 6119985 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 868-14-4(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium bitartrate (868-14-4) |
Safety information for Potassium Bitartrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Potassium Bitartrate
Potassium Bitartrate manufacturer
JSK Chemicals
Rasayan Trading Co.
Canton Laboratories Private Limited
Exemplar
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Potassium hydrogentartrate 98%View Details
Potassium hydrogentartrate 98%View Details -
 Potassium Hydrogen Tartrate CAS 868-14-4View Details
Potassium Hydrogen Tartrate CAS 868-14-4View Details
868-14-4 -
 Potassium Hydrogen Tartarate (SQ) CAS 868-14-4View Details
Potassium Hydrogen Tartarate (SQ) CAS 868-14-4View Details
868-14-4 -
 Potassium Bitartrate Industrial Grade, Purity: 99%View Details
Potassium Bitartrate Industrial Grade, Purity: 99%View Details
868-14-4 -
 Potassium Bitartrate ChemicalView Details
Potassium Bitartrate ChemicalView Details
868-14-4 -
 Potassium Bitartrate, Packaging Type: PacketView Details
Potassium Bitartrate, Packaging Type: PacketView Details
868-14-4 -
 Potassium Bitartrate, For Food / Pharma, Packaging Size: 50 kgView Details
Potassium Bitartrate, For Food / Pharma, Packaging Size: 50 kgView Details
868-14-4 -
 Potassium Bitartrate Chemical, Packaging Type: Bag, Packaging Size: 25 KgsView Details
Potassium Bitartrate Chemical, Packaging Type: Bag, Packaging Size: 25 KgsView Details
868-14-4
