Potassium tartrate
Synonym(s):Potassium bitartrate;Potassium hydrogen tartrate;Tartaric acid monopotassium salt;Potassium hydrogen L -tartrate;L -(+)-Tartaric acid monopotassium salt
- CAS NO.:921-53-9
- Empirical Formula: C4H4K2O6
- Molecular Weight: 226.27
- MDL number: MFCD00013065
- EINECS: 213-067-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:18
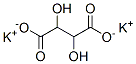
What is Potassium tartrate?
Description
Potassium tartrate, dipotassium tartrate or argol has formula K2C4H4O6. It is the potassium salt of tartaric acid. It is often confused with potassium bitartrate, also known as cream of tartar. As a food additive, it shares the E number E336 with potassium bitartrate.
Chemical properties
white crystalline powder
The Uses of Potassium tartrate
Potassium tartrate is the acid potassium salt of tartaric acid occurring as crystals or powder. It is relatively poorly soluble having a solubility in 100 ml of water at 0.8 g at 25°c and 6.1 g at 100°c. A 1% solution at 30°c has a ph of 3.4. Chemical names are potassium acid tartrate, potassium hydrogen tartrate, and potassium bitartrate. It functions to complex with heavy metal ions and to regulate ph; it can have a gentle laxative action if given at adequate levels. The acidulant is used in chemical leavening to release carbon dioxide which produces the loaf volume. It has limited reactivity in the cold so when used in reduced-temperature batters it has little gas evolution during the initial mixing. At room temperatures, it has a relatively fast reaction rate. It functions as a taste regulator in sugar icing and in controlled crystallization of toffees and fondants by the regulated inversion of sucrose. It is used in baked goods, crackers, candy, and puddings.
The Uses of Potassium tartrate
Manufacture of potassium salts, medicine (cathartic), lab reagent.
Definition
ChEBI: The potassium salt of L-tartaric acid.
Preparation
Potassium tartrate is produced by the reaction of tartaric acid with potassium sodium tartrate (rochelle salt), and potassium sulfate, followed by filtration, purification, precipitation and drying.
Flammability and Explosibility
Not classified
Purification Methods
Recrystallise it from distilled water (solubility: 0.4mL/g at 100o, 0.7mL/g at 14o). [Beilstein 3 IV 1223.]
Properties of Potassium tartrate
| Density | 1.954 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| Flash point: | 200-220°C |
| Decomposition | 200-220 ºC |
| Stability: | Stable. |
| CAS DataBase Reference | 921-53-9(CAS DataBase Reference) |
| EPA Substance Registry System | Butanedioic acid, 2,3-dihydroxy- (2R,3R)-, dipotassium salt (921-53-9) |
Safety information for Potassium tartrate
Computed Descriptors for Potassium tartrate
Potassium tartrate manufacturer
Akshar Exim Company Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Potassium tartrate 98%View Details
Potassium tartrate 98%View Details
921-53-9 -
 Potassium (+)-Tartrate CAS 921-53-9View Details
Potassium (+)-Tartrate CAS 921-53-9View Details
921-53-9 -
 Potassium tartrate CAS 921-53-9View Details
Potassium tartrate CAS 921-53-9View Details
921-53-9 -
 Potassium tartrate, GR 98%+ CAS 921-53-9View Details
Potassium tartrate, GR 98%+ CAS 921-53-9View Details
921-53-9 -
 Cream of Tartar Pure, For Industrial, Grade: Lr GradeView Details
Cream of Tartar Pure, For Industrial, Grade: Lr GradeView Details
868-14-4 -
 Cream Of TartarView Details
Cream Of TartarView Details
921-53-9 -
 Cream Of TartarView Details
Cream Of TartarView Details
868-14-4 -
 Cream of Tartar (COT), Packaging Size: 25KG, Purity: 98-99%View Details
Cream of Tartar (COT), Packaging Size: 25KG, Purity: 98-99%View Details
868-14-4
